, Gila Perk2, Natesa G. Pandian3, Hans-Joachim Nesser4 and Itzhak Kronzon2
(1)
Department of Cardiology, Cardiocentro Ticino, Lugano, Switzerland
(2)
Non-Invasive Cardiology, Lenox Hill Hospital, New York, NY, USA
(3)
Tufts University School of Medicine, Boston, MA, USA
(4)
Department of Medicine Elisabethinen, Teaching Hospital, Linz, Austria
Abstract
Percutaneous occlusion of left atrial appendage (LAA) with specifically designed devices has been proposed for those patients with AF who are ineligible for or non-compliant with anticoagulation therapy.The left atrial appendage has a complex and extremely variable structure with a multi-lobulated configuration.Because the size of occlusion device is strictly dependent on the size and shape of LAA, understanding this complex anatomy has become of paramount relevance for interventionalists involved in percutaneous occlusion of the LAA.Three-dimensional TEE provides a 3D data set of LAA which can be cut and analyzed from a countless number of planes. Additionally, each plane can be observed from multiple perspectives, allowing a more precise assessment of the anatomy of the LAA. Furthermore, a long segment of catheter can be visualized in a single image and the tip of the catheter can be tracked during the procedure. In this chapter we describe the 3D TEE anatomy of the LAA and its role in percutaneous closure of LAA.
Electronic supplementary material
The online version of this chapter (doi:10.1007/978-1-4471-4745-9_6) contains supplementary material, which is available to authorized users.
Atrial fibrillation (AF) is the most common clinically significant cardiac arrhythmia, accounting for approximately one third of hospitalizations for cardiac rhythm disturbance. The prevalence of AF is estimated at 0.4–1.0 % of the general population, but it increases with age. Though AF occurs in less than 1 % of adults 55 years of age or younger, its prevalence is at least 9 % in individuals age 80 or older.
AF is characterized by rapid and uncoordinated depolarization wave fronts in the atrial tissue, leading to atrial tachyarrhythmia, reduced intra-atrial flow velocity, and blood stasis. Stasis is particularly pronounced in the left atrial appendage (LAA), eventually causing thrombus formation. Hemodynamic derangement and risk of systemic emboli are the two most harmful complications of AF. Accordingly, medical treatment focuses on control of rate and/or rhythm and the prevention of thromboembolism. Long-term anticoagulation therapy is the cornerstone of treatment for primary and secondary prevention of thromboembolic events. Such therapy has several drawbacks, however, including the difficult balance between efficacy and risk of bleeding, requiring frequent blood tests (subtherapeutic levels are associated with higher risk of embolism, and supratherapeutic levels, with a risk of major bleeding), drug interactions, and risk of major bleeding in case of traumatic events. The new oral direct inhibitors of thrombin and factor Xa appear to have a wider therapeutic window, fewer drug interactions, and no need for frequent blood tests. Some of these are currently available for use, having been shown to be noninferior to warfarin in the prevention of strokes in patients with AF. Nonetheless, a bleeding risk remains with any anticoagulant therapy, and the risk of cerebrovascular events, though reduced, is not totally abolished with anticoagulation. Moreover, a significant percentage of patients with AF (from 10 to 30 %, according to different series) have contraindications to anticoagulation.
Because more than 90 % of thrombi are formed in the LAA, isolation of the LAA from the systemic circulation has gained interest in the past few years as a nonpharmacologic alternative to anticoagulation. Surgical ligation or excision of the LAA in patients with AF who undergo valvular or coronary artery bypass graft surgery is appealing, although its impact on stroke prevention has never been systematically studied. Surgical exclusion as a stand-alone procedure is far less attractive, however, as the risk of open heart surgery would exceed any potential benefit of LAA exclusion.
Percutaneous occlusion of the LAA with specifically designed devices has been proposed for patients with AF who are ineligible for anticoagulation therapy or are noncompliant with it. Clinical trials have shown that this procedure is noninferior to anticoagulation with respect to the primary endpoint of stroke, cardiovascular death, or systemic embolism.
The LAA has a complex and extremely variable structure, with a multilobulated configuration. Muscular bundles (pectinate muscles) divide the main cavity into cubicles of variable sizes. Secondary cavities (lobes) may be present, extending out from the main cavity in different directions. Because the size of an occlusion device is strictly dependent on the size and shape of the LAA, understanding this complex anatomy has become of paramount relevance for interventionalists involved in its percutaneous occlusion.
Several imaging techniques, including CT, MRI, intracardiac echocardiography, and two-dimensional transoesophageal echocardiography (2D TEE), have been used to visualize the LAA. Because of its versatility, high frame rate, and spatial resolution, 2D TEE remains the primary imaging modality to exclude LAA thrombus. It is performed before LAA occlusion to define the maximum LAA width and the depth of the dominant cavity. Two-dimensional TEE is also used to guide the procedure (along with fluoroscopy and angiography); it has been shown to be particularly valuable in directing transseptal puncture, assessing the position and orientation of the device in situ, and confirming total obliteration of the appendage. However, because of its tomographic nature, 2D TEE requires several planes and multiple probe manipulations to adequately assess the complex anatomy of the LAA and its spatial relationship with surrounding structures. Moreover, the entire length of a catheter cannot be visualized in a single plane, and the catheter’s tip may be difficult to follow.
Three-dimensional (3D) TEE provides a 3D data set of the LAA, which can be cut and analyzed from a countless number of planes. Additionally, each plane can be observed from multiple perspectives, allowing a more precise assessment of the anatomy of the LAA. Furthermore, a long segment of catheter can be visualized in a single image, and the tip of the catheter can be tracked during the procedure. This chapter describes the 3D TEE anatomy of the LAA and the role of 3D TEE in preparing for and performing percutaneous closure of the LAA.
6.1 Three-Dimensional TEE Anatomy of the Left Atrial Appendage
There are several reasons why 3D TEE is particularly appropriate for LAA visualization. First, the LAA is positioned close to the transducer (at roughly the same depth as the mitral valve), providing images of exquisite quality owing to the high line density. Second, from the atrial perspective, the entire circumference of the LAA orifice can be visualized en face (this information cannot be obtained with 2D TEE). Third, the small size of the LAA allows use of the “zoom modality” with a narrow angle, providing a relatively high frame rate (up to 25 Hz), while maintaining optimal image quality.
In individuals with normal left atrial dimensions, 3D TEE imaging of the LAA from an atrial perspective usually shows an elliptical orifice. The orifice is located between the left upper pulmonary vein (from which it is separated by the left lateral ridge) and the mitral valve (Fig. 6.1a). From this perspective, pectinate muscles, if prominent, can be visualized as narrow bars crossing the LAA perpendicularly to its long axis (Fig. 6.1b). In patients with persistent or permanent AF, the orifice becomes less elliptical and more rounded, possibly reflecting the stretch in an enlarged left atrium.
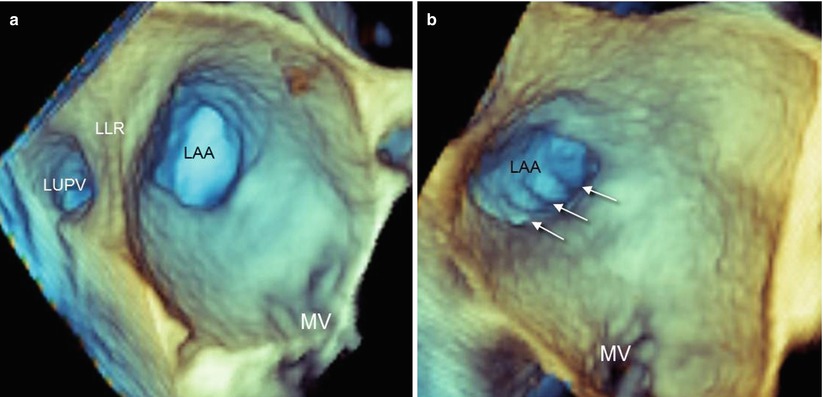

Fig. 6.1
(a) Left atrial appendage (LAA) orifice opening into the left atrium. The orifice is located between the left upper pulmonary vein (LUPV) and the mitral valve (MV). The left lateral ridge (LLR) separates the LUPV from the LAA. In normal individuals, the orifice is elliptical. (b) Prominent pectinate muscles (arrows) can be seen running perpendicularly to the long axis of the LAA
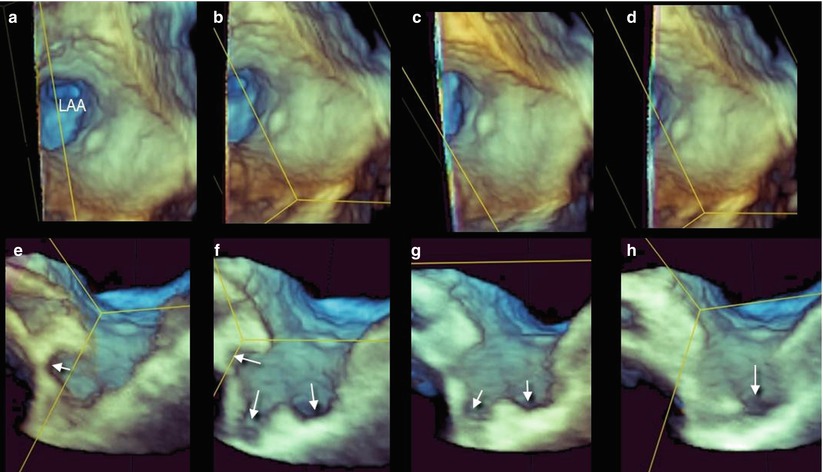
A larger view from the same atrial perspective may show the spatial relationship with surrounding structures (left lateral ridge, left upper pulmonary vein, left pulmonary artery, and mitral valve) (Fig. 6.2). From the en face view of the LAA orifice, longitudinal cropping and proper rotation may reveal the LAA with its lobes in long-axis view (Fig. 6.3). However, because the lobes may be located at different depths, one single longitudinal plane may not show all the lobes of the LAA. Sequential cropping allows an accurate assessment of the LAA anatomy, exposing lobes positioned at different depths and directions (Fig. 6.4).
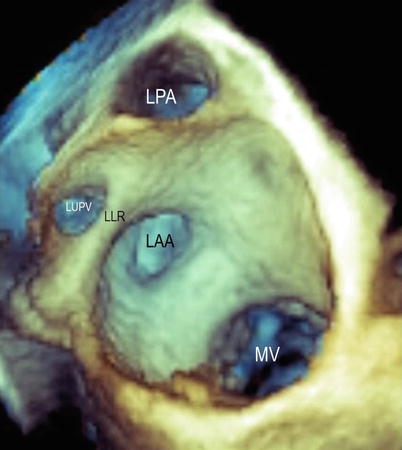
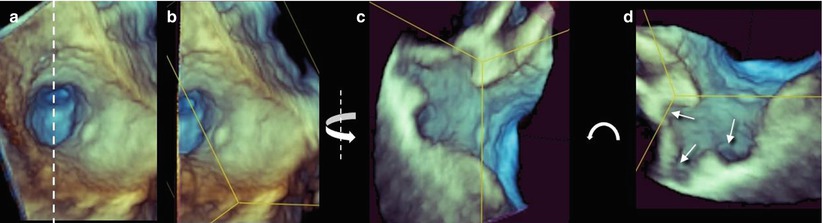

Fig. 6.2
A panoramic view from the atrial perspective shows the spatial relationship between the left atrial appendage (LAA), left pulmonary artery (LPA), left upper pulmonary vein (LUPV), and mitral valve (MV). LLR left lateral ridge

Fig. 6.3
(a) The en face view of the left atrial appendage (LAA) orifice. The dotted line shows the plane passing through the center of the orifice, dividing the appendage into two halves. (b) The left half of the image has been removed. (c) The remaining right half is rotated on the Y plane (curved arrow) to obtain a longitudinal view. (d) The image is further rotated on the Z plane to obtain an anatomically correct orientation. The arrows show different lobes at different depths