Patients with recurrent head and neck cancer have poor quality of life and suffer dismally from debilitating symptoms. Ablative techniques offer patients an alternative, minimally invasive treatment option. As a palliative treatment, they improve quality of life with decreased pain, improved function and appearance. In addition, there is a reduction in tumor bulk and analgesia requirements. Advantages include a reduction in procedural cost, avoidance of complex repetitive surgeries, and an ability to visualize the treated area at the time of the procedure. Ablation therapies are an evolving and exciting treatment option in the head and neck, but a consensus on appropriate indications is currently unclear.
Image-guided ablation therapies are an emerging minimally invasive treatment modality for the treatment of head and neck tumors. These techniques are predominantly used as a palliative treatment of pain and local tumor control in the setting of recurrent head and neck cancers. In patients with a head or neck tumor, wide surgical resection offers the best cure. Unfortunately, the majority of patients presents with locally advanced disease and are not good surgical candidates because of their disease extent or poor functional status. In these patients, minimally invasive ablation treatments potentially offer improved quality of life, decreased tumor burden and may increase survival.
Tumor ablation results in the focal destruction of solid tumor by eradicating all viable tumor cells in the area. The head and neck is a unique location with its own inherent risks related to the close proximity of the surrounding anatomical structures. Percutaneous ablation therapies offer potential advantages with minimal damage to surrounding tissue with preservation of surrounding tissue function.
Ablation techniques are most often used in the setting of recurrent disease with a history of previous surgery and or radiation therapy. In this setting, treatments such as repeat surgery, external beam radiation and systemic chemotherapy have poor results in terms of survival and quality of life. Scar tissue and loss of the normal anatomical planes makes recurrent surgery challenging with high rates of complications. These common treatments are often toxic with significant effect on swallowing function, speech, cosmetic appearance and poor wound healing.
Ablation techniques such as radiofrequency ablation (RFA) and cryotherapy are in widespread use and have been shown to be successful in the setting of various tumors involving the bone, lung, prostate, kidney, and liver. As compared with repetitive invasive surgery, ablation techniques offer a significant reduction in morbidity and mortality at a much lower procedural cost. Ablations are often carried out as outpatient procedures or with a short hospital stay. There is a minimal incision and no significant blood loss.
Ablative techniques offer an additional treatment option for patients with head and neck cancer. It is critical that these techniques, whether used alone or in combination with other therapies, are used as a consensus derived, multidisciplinary approach to cancer.
Tumors of the head and neck: ablation techniques
As in many medical disciplines, patient selection is critical. Use of ablative techniques in the head and neck have been reported in the literature in the setting of squamous cell carcinoma, tumors of the oral cavity, paranasal sinuses, thyroid tumors, adenoid cystic carcinoma of the salivary glands, solitary fibrous tumors, and recurrent melanoma. Primary surgical resection, modified neck dissections and external beam radiation remain the pillars of primary treatment for these conditions. Ablative techniques have been shown to be effective as primary treatment in certain subgroups of patients—such as those who are excluded from surgical cure because of significant heart, lung, or liver disease. However, the role of ablative techniques in the setting of primary treatment is currently unproven and all patients should be offered gold standard, first line therapy prior to an ablation procedure.
Diagnostic work-up and imaging
Clinical evaluation and review of all relevant imaging is imperative prior to any ablation procedure. Patient expectations and clear treatment goals need to be defined. Critical appraisal of all cross-sectional imaging with particular attention to tumor extent and proximity of delicate surrounding structures is essential. Renal function, platelet counts, and coagulation factors are all reviewed. In cases of recurrent thyroid carcinoma, endoscopic examination of the vocal cords is warranted.
Depending on the tumor location, an ablation probe can be inserted percutaneously, transorally, or by an endoscopic guided route. Ablation procedures are commonly performed under CT or ultrasound guidance. The choice of the guiding modality depends on tumor location and operator preference. The key to all ablation techniques is the precise positioning of the ablation probe within the tumor. Multiple probes or overlapping ablation segments ensure eradication of all local tumor cells. Ultrasound is commonly used in the setting of thyroid tumors ( Fig. 1 A–D ). In addition to allowing excellent real-time visualization, ultrasound is better at visualizing ablative zones than CT in the setting of RFA.
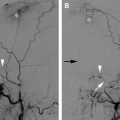
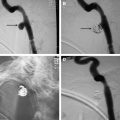
CT also provides precise anatomic visualization of the tumor. The use of contrast can aid in tumor visualization and in determining the relationship of the tumor to the adjacent vascular structures. CT fluoroscopy can be used to aid in accurate probe insertion and avoid inadvertent puncture of any vessel or the aerodigestive tract ( Fig. 2 ). Contrast-enhanced CT after the ablation may lead to identification of residual enhancing tumor that can be further ablated in the same treatment setting. There is a role for interventional MR imaging guidance in this field as the thermal imaging capability can define heat distribution and thus allow reduction of inadvertent nerve or other organ injury.
Diagnostic work-up and imaging
Clinical evaluation and review of all relevant imaging is imperative prior to any ablation procedure. Patient expectations and clear treatment goals need to be defined. Critical appraisal of all cross-sectional imaging with particular attention to tumor extent and proximity of delicate surrounding structures is essential. Renal function, platelet counts, and coagulation factors are all reviewed. In cases of recurrent thyroid carcinoma, endoscopic examination of the vocal cords is warranted.
Depending on the tumor location, an ablation probe can be inserted percutaneously, transorally, or by an endoscopic guided route. Ablation procedures are commonly performed under CT or ultrasound guidance. The choice of the guiding modality depends on tumor location and operator preference. The key to all ablation techniques is the precise positioning of the ablation probe within the tumor. Multiple probes or overlapping ablation segments ensure eradication of all local tumor cells. Ultrasound is commonly used in the setting of thyroid tumors ( Fig. 1 A–D ). In addition to allowing excellent real-time visualization, ultrasound is better at visualizing ablative zones than CT in the setting of RFA.
CT also provides precise anatomic visualization of the tumor. The use of contrast can aid in tumor visualization and in determining the relationship of the tumor to the adjacent vascular structures. CT fluoroscopy can be used to aid in accurate probe insertion and avoid inadvertent puncture of any vessel or the aerodigestive tract ( Fig. 2 ). Contrast-enhanced CT after the ablation may lead to identification of residual enhancing tumor that can be further ablated in the same treatment setting. There is a role for interventional MR imaging guidance in this field as the thermal imaging capability can define heat distribution and thus allow reduction of inadvertent nerve or other organ injury.
Radiofrequency ablation
The aim is to ablate all viable tumor cells with a small surrounding margin of normal tissue, similar to the concept of clear surgical resection margins. Sterile grounding pads are evenly placed in a position that is easy and convenient to check, they are optimally placed equidistant from the target ablation site to allow equal grounding of thermal energy, usually on both thighs. This helps avoid skin burns. Delicate surrounding visceral or vascular structures can be pushed away from the ablated zone by means of a hydrodissection technique in which a small needle is carefully placed between target tissue and the surrounding vital structures. Intervening space can be increased by the introduction of an insulator such as sterile water, dextrose, air, or carbon dioxide. Saline is a conductor and may increase the size of the ablation zone. An RFA probe consists of an insulated shaft and an active tip. Probes come in varies sizes with multiple tip lengths and configurations. Deployable distal tips with multiple tines are used for large bulky tumors ( Fig. 3 ). These complex shapes allow for large reproducible regions of ablation. Point tips are often used for smaller lesions. Through the probe, there is transmission of low-voltage alternating current that creates ionic agitation, heating, intratumoral hyperthermia, and tumor necrosis. This is referred to as focal coagulative necrosis. Both current and heat decrease exponentially as the distance increases from the active tip of the probe. Ablation temperatures reach 50°C to 100°C. There is a cytotoxic threshold temperature for the tumor cells. Most malignant cells are rapidly destroyed above 50°C. The aim is for the entire tumor to be subject to a cytotoxic temperature. If the tissue surrounding the tip of the probe is overheated, it will vaporize and char. This will decrease the energy absorbed and reduce the size of the surrounding ablation. When tissue reaches in excess of 100°C, it will vaporize with resultant reduced ablation of the surrounding tissue. The impedance or temperature at the probe tip is monitored and the output of the generator is adjusted to prevent vaporization and charring. To avoid charring, probes can have a cooled tip with chilled saline circulating in the shaft of the probe.