Author
Patient number
Primary cancer
R0 resection
MST (months)
Survival (%)
1-year
3-year
5-year
Choti et al. [14]
226
Colorectal
N/A
46
N/A
N/A
40
Sarmiento et al. [19]
170
Neuroendocrine
N/A
N/A
N/A
N/A
61
Takemura et al. [20]
145
Non-colorectal
N/A
41.8
83.9
55.4
41.0
Non-neuroendocrine
Amano R et al. [15]
117
Colorectal
N/A
58
92.3
60.0
46.1
Takemura et al. [21]
64
Gastric
55
34
84
50
37
Niu et al. [22]
60
Ovarian
54
39
N/A
N/A
30
Kostov et al. [18]
42
Breast
35
60
84.61
64.11
38.45
Cheon et al. [23]
41
Gastric
N/A
17
75.3
31.7
20.8
3.2.2 Minimally Invasive Treatment
A small percentage of patients are surgical candidates, and patients will live longer with cancer; for these reasons, comorbid conditions become a major factor in selecting patients for surgical resection. This is particularly important in those patients with multiple comorbidities, or more advanced disease. Besides, the high rate of recurrence of liver metastases, affecting 53–68 % of patients, would require repeated resection, which can be tolerated by only a minority of patients with adequate functional future liver remnant and good liver function. For the above reasons, other alternatives that are effective and minimally invasive and repeatable techniques for the treatment of liver metastases are badly in demand. Clinical outcomes of minimally invasive treatment of hepatic metastases are summarised in Table 3.2.
Table 3.2
Minimally invasive treatment of liver metastases studies since 2001
Author | Technique | Patient number | Primary cancer | Mean size (cm) | CA (%) | LTP (%) | MST (m) | Survival (%) | ||
|---|---|---|---|---|---|---|---|---|---|---|
1-year | 3-year | 5-year | ||||||||
Vogl et al. [37] | TACE | 208 | Breast | N/A | N/A | N/A | 18.5 | 69 | 33 | N/A |
Albert et al. [38] | TACE | 121 | Colorectal | N/A | N/A | N/A | 27 | N/A | N/A | N/A |
Ruers et al. [39] | Cryoablation | 30 | Colorectal | N/A | N/A | 9 | 32 | 76 | N/A | N/A |
Mala et al. [40] | Cryoablation | 19 | Colorectal | N/A | N/A | 44 | N/A | N/A | N/A | N/A |
Gillams et al. [41] | RFA | 309 | Colorectal | 3.7 | N/A | N/A | N/A | N/A | N/A | 34 |
Solbiati et al. [42] | RFA | 117 | Colorectal | 3.2 | 98 | 39 | 36 | 93 | 69 | 46 |
Machi et al. [43] | RFA | 100 | Colorectal | N/A | N/A | N/A | 28 | 90 | 42 | 30.5 |
Liang et al. [44] | MWA | 74 | Colorectal (28) | 3.12 | N/A | 14 | N/A | 91.4 | 46.4 | 29 |
Lorentzen et al. [45] | MWA | 39 | Colorectal (31) | N/A | 100 | 9.6 | N/A | N/A | N/A | N/A |
3.2.2.1 TACE
TACE is a local, catheter-based, minimally invasive therapeutic option for unresectable liver tumours and is defined as a selective administration of chemotherapy usually combined with embolisation of the vascular supply of the tumour [46]. As a combination of local arterial application of chemotherapeutic drugs with additional usage of embolisation particles, TACE is an effective and recommended treatment [47]. TACE was successfully applied in patients with liver metastases from colorectal carcinoma [38, 47]. Duan et al. reported that the combined treatment of TACE and systemic chemotherapy may prolong survival for liver metastases in breast cancer after mastectomy. Albert et al. also suggested that chemoembolisation with cisplatin, doxorubicin, mitomycin C, Ethiodol and polyvinyl alcohol provides local control of metastatic colorectal carcinoma to the liver during or after standard systemic therapy [38]. In selected patients with liver metastases from ovarian cancer, TACE is an effective palliative treatment in achieving local control, and from the start of TACE, the 1-, 2- and 3-year survival rates were 58, 19 and 13 %, respectively [10]. For the unresectable hepatic metastases of breast cancer, the 1-, 2- and 3-year survival rates after TACE with mitomycin C and gemcitabine were 69, 40 and 33 % [37]. Although considered to be relatively safe, TACE has been associated with several disadvantages including drug resistance, recurrence of marginal tumour and bad therapeutic effect in tumour absence of blood supply as well as relatively high complication rate. Some risk factors have been associated with an increase in complications after TACE treatment, the most known being a poor hepatic reserve with increased serum bilirubin levels. Around 75 % of patients had postembolisation syndrome (fever, pain, nausea) and the total complication rate per procedure was about 9.1 % [48]. For these reasons, TACE combined with local physical therapy including radiofrequency ablation (RFA), microwave ablation (MWA), ethanol ablation, cryoablation and laser ablation will achieve better tumour control than monotherapy.
3.2.2.2 Cryoablation
Several local tumour ablative techniques may offer an alternative therapeutic option in case of liver metastases. Cryoablation, the use of low temperatures to induce local tissue necrosis, was among the first line of the thermal ablative techniques widely used [49]. In the past, cryosurgery has proven to be a relatively safe and effective ablative technique [39, 50]. A median survival of 13–32 months has been reported following cryoablation of CLM [39, 51, 52]. However, a large study of cryoablation of colorectal metastases reported a local recurrence rate of 33 % after a median follow-up of 22 months [53]. Intra-arterial chemotherapy was given to 91 % of the patients. Mala and colleagues reported that 44 % of the patients developed local tumour recurrence during follow-up [40]. New intra- or extrahepatic recurrences of CLM are found in 20–67 % and 40–46 % of the patients, respectively [53]. Local tumour recurrences are more frequent following freezing of large metastases (>3 cm) and metastases located close to large vessels [52, 53]. The complications of hepatic cryoablation seem to be related to the extent. Myoglobinaemia is a special complication correlated to the amount of tissue frozen [54]. Serum myoglobin returns to normal within three days, but may cause renal failure if adequate urinary output is not maintained. Cryoshock is a potentially lethal complication following freezing of large liver volumes [55]. The syndrome is characterised by multiorgan failure and resembles a condition of septic shock without evidence of systemic sepsis.
3.2.2.3 RFA
In recent years, with the additional refinements to the design and power of the equipments, RFA has emerged as the most popular tool for the destruction of hepatic and other malignancies. Current RFA uses a high-energy alternating current applied via an exposed electrode placed directly into a target lesion. Initially, RFA has been applied to treat patients with tumour limited to the liver who did not meet the criteria for surgical resectability [56–58]. Currently, RFA is offered to those who cannot undergo resection because of inadequate liver reserve, coexisting morbidity or patient choice and is performed with a curative intent as in surgical resection. There are increasing reports evaluating the use of RFA in the treatment of liver metastases of colorectal cancer patients with the 5-year survival rate from 34 to 46 % [41–43]. There are a small number of retrospective reports on RFA for breast cancer liver metastases (BCLM). In 2001, Livraghi et al. reported on 24 patients with 64 hepatic breast metastatic foci treated with RFA [59].Complete necrosis by imaging was found in 92 %, and 58 % went on to develop new sites of metastases during follow-up. Metastatic breast cancer is a systemic disease, and most of the patients are receiving concurrent systemic chemotherapy. For this reason, both ablation and resection for BCLM should be considered an adjuvant therapy rather than a substitute for systemic therapy. Based on this theory, RFA may ultimately prove to be as effective as surgical resection for debulking of BCLM. The complication rates for percutaneous RFA of hepatic tumours in 3,670 patients are 7.2 % [60]. Overall, the frequency of major complications of percutaneous RFA ranges from 0.6 to 8.9 % [61]. RFA has a potential to achieve the same overall and disease-free survival rate as surgical resection for patients with liver metastases, whilst causing fewer side effects.
3.2.2.4 MWA
MWA therapy is a relatively new technique that can be applied to different types of tumour. In the early 1980s, a microwave device similar to the “Bovie knife” was firstly used to coagulate the tissue during hepatic resection [62, 63]. Over the next 20 years, this technique evolved rapidly and was used in many of the clinical applications, such as the treatment of primary hepatic malignancies and secondary hepatic malignancies, kidney tumours as well as benign thyroid nodule. One of the main benefits of MWA is that microwaves can propagate through desiccated and charred tissue formed during ablation to form a large ablation zone. For this reason, compared with RFA the size and shape of the MWA zone may be more consistent and less dependent on the heat-sink effect from vascular structures or large bile in proximity of the lesion [64, 65]. Iannitti et al. [66] treated 11 patients with BCLM and showed that after the 19-month follow-up, 36.4 % of the patients were alive with no disease, 9.1 % were alive with disease and 54.5 % had died of disease [66]. Abe et al. [67] used magnetic resonance imaging-guided MWA on 11 nodules in 8 patients with BCLM. No mortality or major complications occurred as a result of the procedure [67]. Lorentzen et al. [45] and our team (2003) have reported the prognostic factors that can affect survival after MWA therapies [45, 68]. Stättner et al. compared the efficacy of surgical MWA with or without resection for CLM [69]. Twenty-eight underwent combined MWA and resection, whilst 15 underwent MWA as the sole treatment modality. At a median follow-up of 15 months, local treatment failure was observed in 4 % of ablated lesions. Three-year OS was 36 % for MWA group, compared to 45 % for the combined ablate/resect group with 3-year DFS of 32 and 8 %, respectively.
Since 1998, our team has gained rich experience in managing many kinds of solid malignancies including primary liver cancer [70] and hepatic metastasis [68]. In 2003, our study showed that the cumulative survival rate of all 74 patients with liver metastases (11 patients had breast cancer) was 29 % at 5 years. Patient age, sex and site of primary malignancies were not related to prognosis, whereas tumour grade, number of metastases and local recurrence or new metastasis affected survival as single independent factors. Multivariate analysis revealed that tumour grades, number of metastases and local recurrence or new metastasis had a significant effect on survival. In the past few years with the additional refinements to the power and design of the MW system including water-cooled-shaft antenna, thermal monitoring system and other assistive technologies, more and more patients with primary and secondary hepatic malignancies benefit from this thermal technique.
3.2.3 Indications
For the patients with metastatic tumours (single lesion of 5 cm or smaller, five or fewer multiple lesions with a maximum diameter of 3 cm or less and absence of apparent vascular or biliary invasion) and for the patients that do not come up to the above requirements, standard chemotherapy or targeted molecular medicine was suggested to use and a second evaluation should be performed before MWA. There is no severe dysfunction of the liver, kidney, heart and central nervous system; blood coagulate function is normal or close to normal; refusal of surgery or unresectable central type or deep-seated lesions. For the large lesions with rich blood supply, TACE treatment should be performed firstly, and the residual tumour could be destroyed with the guidance of contrast-enhanced ultrasound. For those with too much or too large tumour lesion, both surgery and systemic chemotherapy may have no apparent effect; MWA can reduce the tumour load to slow down the tumour progress for pain relief and life extension.
3.2.4 Contraindications
In diffuse liver metastasis with apparent vascular or biliary invasion, oversized tumour lesion and the ablation zone should reach to about one third of the whole liver or larger. There is severe dysfunction of major organs, irremediable coagulation disorders (platelet count is less than 30 × 109/L, prothrombin time is 30 s or more and prothrombin activity is less than 40 %) and haematologic diseases with severely abnormal blood routine index.
3.2.5 Equipments
The same as stated in the above chapters.
3.2.6 Techniques
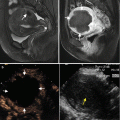
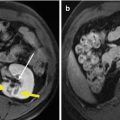
Most of the techniques of MWA are the same with liver metastases as with primary liver cancer. However, there are many differences in pathological characteristic between liver metastases and primary liver cancer; accordingly, during MWA of liver metastases, special technical skills are applied according to the types of the tumour lesion and the source of primary tumour. In order to destroy the tumour in treating primary liver cancer, one to three thermal couples are placed at different sites 0.5 cm outside the tumour. However, for liver metastases, the safe boundary should be enlarged to 1 cm outside of the tumour. Specially, for the cystic or cystic-solid mixed liver metastases from ovarian cancer and nasopharyngeal cancer, before MWA hydatid fluid should be aspirated and appropriate amount of dehydrated alcohol be injected into the cyst for at least 3 min, and then MWA is performed to destroy the solid part of the tumour lesion (Fig. 3.1).