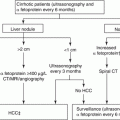
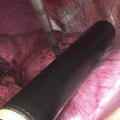
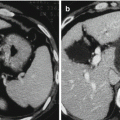
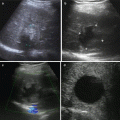
Fig. 7.1
Grounding pad burns after RFA
7.3.3 Intraperitoneal Bleeding
Bleeding complications are more likely to happen in patients with HCC due to their underlying liver diseases. The bleeding can present in the form of subcapsular hematoma, abdominal wall hematoma, intrahepatic parenchymal hematoma, or even hemoperitoneum [14–16]. It is important to select a needle electrode path that traverses sufficient normal liver parenchyma to avoid hepatic vessels while positioning the needle electrode and to perform cauterization of the needle electrode track after ablation. Increasing abdominal pain following the procedure is generally the most common symptom. Repeat hemoglobin level, ultrasound, or CT scan confirms the diagnosis. Although most minor bleeding after RFA stops spontaneously, close clinical evaluation of a patient’s vital signs and laboratory tests immediately after ablation are essential for early detection and proper management of this potentially life-threatening complication (e.g., fluid resuscitation, blood transfusion, correction of coagulopathy). Venous bleeding sometimes stops by itself. Uncontrolled arterial bleeding or pseudoaneurysm formation needs to have transfemoral hepatic arterial branch embolization to control the bleeding. If bleeding is not controlled by non-surgical means, surgical intervention is needed.
7.3.4 Hepatic Failure
Liver failure is also a potentially fatal complication, especially in patients with cirrhosis whose liver function is already impaired. Similar to post-hepatectomy patients, there are multiple risk factors for the development of this severe complication, such as insufficient liver functional remnant, vascular thrombosis, major biliary injury, liver abscess, or sepsis due to intestinal perforation.
7.3.5 Intrahepatic Abscess
Secondary bacterial contamination of the ablated hepatic parenchyma may lead to abscess formation. The presence of biliary-enteric communication at the time of the procedure has been noted to significantly increase the risk of abscess formation [17]. It can be difficult to differentiate fever as a component of the post-ablation syndrome from that secondary to an intrahepatic abscess. This difficulty may delay the diagnosis. The possibility of abscess formation should be considered if the fever pattern is high and swinging or lasts longer than 1 week because fever in the post-ablation syndrome usually is low grade and lasts less than 1 week. Most abscesses can be successfully managed by simple aspiration or percutaneous catheter drainage coupled with adequate antibiotics coverage.
7.3.6 Bile Duct Injury
Biliary tract damage includes biliary stricture; bilomas; and, rarely, bilio-peritoneum and bilio-pleural fistula. The proximity of liver tumor to the right or left hepatic ducts, or sectoral bile ducts, increases the risk of biliary stricture or biliary fistula due to thermal induced necrosis and/or direct ductal puncture. Most of these changes have no clinical significance with the patient being asymptomatic, and major complications requiring additional treatment are rare. While biliary stricture has no clinical significance when it is located peripherally, it is sometimes troublesome when located centrally in the major bile ducts. Bile duct cooling using an endoscopic nasobiliary drainage (ENBD) tube during RFA for HCC close to major bile ducts has been reported to be safe and feasible [18–20]. This technique increases the indications of RFA in difficult cases. Another complication is hemobilia, which is caused by the simultaneous puncture of the biliary tract and a vessel. The most common symptoms are abdominal pain, hematemesis, and melena.
RFA of tumors adjacent to the gallbladder is often accompanied by a high risk of gallbladder perforation or acute cholecystitis due to thermal injury. Therefore, when treating tumors adjacent to the gallbladder, an open or laparoscopic technique provides the chance to perform prophylactic cholecystectomy, thus decreasing the risk.
7.3.7 Vascular Thrombosis
Portal vein thrombosis, hepatic vein thrombosis, hepatic artery damage, and pseudoaneurysm represent this group of complications. Heat-related vascular thrombosis is only observed in vessels smaller than 3 mm and has no symptomatic consequences. Larger caliber vessels are protected by the continuous cooling effect of blood flow. Most of these thromboses are asymptomatic even in large vessels, and no further therapy is required. In general, thrombi within portal and hepatic veins require no specific therapy if liver function is unaffected. They are caused by heat damage to the endothelial cells of the portal or hepatic vein, leading to platelet aggregation and subsequent thrombosis.
7.3.8 Gastrointestinal Tract Injury
Liver tumors are sometimes adjacent to the stomach, ascending colon, and duodenum. When a liver tumor is close to the gastrointestinal tract, the gastrointestinal tract should be displaced away from the tumor to avoid thermal injury from RFA. Tumors that lie adjacent to the gastrointestinal tract may be protected by changing the patient’s body position, interposition of induced ascites, or aspirating gas inside the bowel [21]. If safe displacement of the gastrointestinal tract is not feasible, laparoscopic or open approach of RFA should be considered. Early diagnosis and adequate treatment of thermal injury to the gastrointestinal tract are essential since it may lead to death.
7.3.9 Thoracic Complications
Pneumothorax, hemothorax, pleural effusions, and pneumonia are in this group of complications. For liver tumors which are located near the hepatic dome, the diaphragm is particularly exposed to the risk of thermal injury; the consequences are generally limited to a reactive pleural effusion or to a more intense and prolonged pain syndrome. Delayed diaphragmatic hernia can occur as a result of this thermal injury, and these patients can be asymptomatic or present with acute bowel strangulation [22, 23].
Several authors have also described hemothorax as a complication of percutaneous RFA. It is less frequent than intra-abdominal bleeding. It usually occurs when an intercostal approach is used to carry out RFA for patients with tumors in the right liver, with injuries to intercostal arteries. Diaphragmatic muscle hemorrhage has also been reported during trans-diaphragmatic approach of RFA using artificial hydrothorax for subdiaphragmatic liver malignancies [24]. Chest pain and dyspnea are the most common symptoms. Ultrasound, CT scan, and chest X-ray confirm the diagnosis.
7.3.10 Tumor Seeding
Tumor seeding in the needle tract has been reported with an incidence varying from 0.6 % to 4 % [25–30]. Viable tumor cells adherent to a biopsy needle or a RFA needle electrode which drop off during needle extraction, tumor cells which are carried into the needle tract during bleeding, or tumor cells which are forced into the needle tract by intratumoral hyperpressure created by the RFA process are the mechanisms that explain tumor seeding (Fig. 7.2). Decreasing the number of punctures and transversing a large amount of liver parenchyma before entering the tumor may prevent this complication. Indirect punctures through an area of healthy liver parenchyma and coagulation of the needle tract are recommended to reduce the risk of bleeding and tumor implantation, especially for subcapsular lesions. Other risks factors reported are poor tumor differentiation, subcapsular location (where heating of the needle tract is not possible), previous biopsy before RFA, and multiple needle insertions. Therefore, an optimal and meticulous first attempt in the needle electrode positioning is desirable.
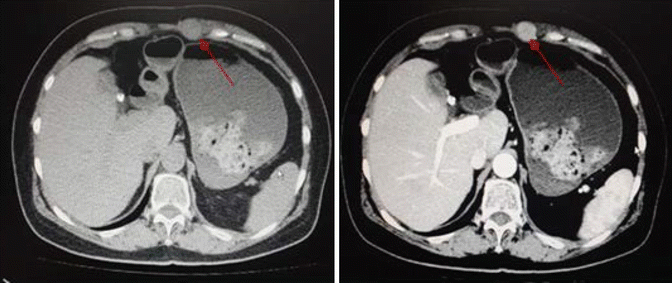

Fig. 7.2
Tumor (arrows) seeding to abdomen wall after RFA
7.4 Conclusion
Careful patient selection, choice of the most appropriate imaging modality and approach, followed by early detection and appropriate management should a complication occur help to minimize the incidence of complication and the degree of morbidity of RFA. Clinicians utilizing RFA to treat liver tumors must be aware that treatment-related complications can develop even months after the treatment.