Introduction
Peripheral vascular disease continues to be a leading cause of death and disability in the United States and the Western world.1 Advancements in imaging modalities have enhanced our ability to better understand both the anatomy and pathophysiology of vascular diseases. Health care practitioners with a clear understanding of the available imaging modalities and their utilization will be better positioned to optimize outcomes in patients with peripheral vascular diseases.
In this chapter, we present carotid, aortic, and lower extremity atherosclerotic peripheral vascular disease cases with computed tomography angiography (CTA) images that illustrate key points in diagnosis and treatment.
The recent and rapid advancement of computed tomography (CT) scanners from single-row to multidetector-row scanners has greatly enhanced our ability to image the body’s vasculature. Wider detector arrays allow for more rapid image acquisition, using less contrast and less radiation dose. Improved spatial resolution of the new generation of scanners also provides more details of the vascular anatomy including plaque morphology characterization. Postprocessing of these rapidly acquired images with three-dimensional (3D) workstations allows viewing of images in multiple planes and with multiple viewing techniques.
CTA imaging has several advantages in peripheral arterial disease: (1) excellent spatial resolution; (2) exceptional sensitivity and specificity; (3) ability to assess postocclusion anatomy; (4) short examination time; (5) ability to accurately assess vasculature that has been revascularized (stented or bypassed); (6) imaging in multiple planes; (7) lack of interference with metal, including cardiac devices; and (8) patient preference and comfort. There is also the added benefit that CTA and magnetic resonance (MR) can potentially allow for the diagnosis of nonvascular abnormalities that may be clinically significant.
Case 1: Carotid Artery Disease
A 68-year-old man presents to the office of his primary care physician after experiencing several episodes of transient right hand paresthesias that last for less than 1 hour. He has a history of hypertension, dyslipidemia, and known coronary artery disease with three-vessel bypass 5 years prior to presentation. Physical exam is without focal neurologic deficits but does reveal bilateral carotid bruits. Blood pressure is 95/55 mm Hg. A duplex ultrasound (DUS) of the neck is ordered for evaluation.
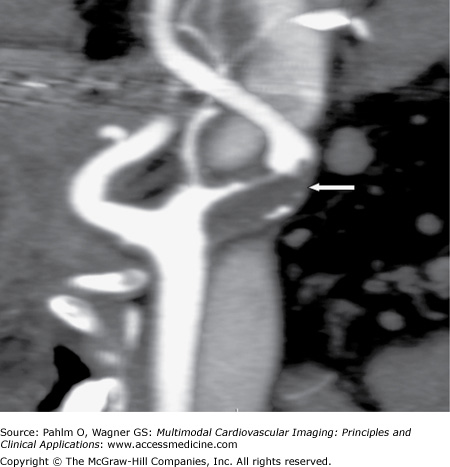
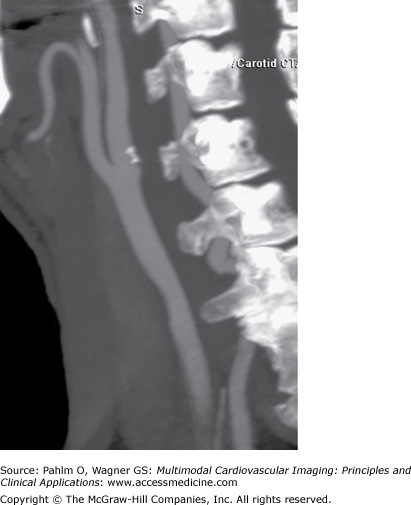
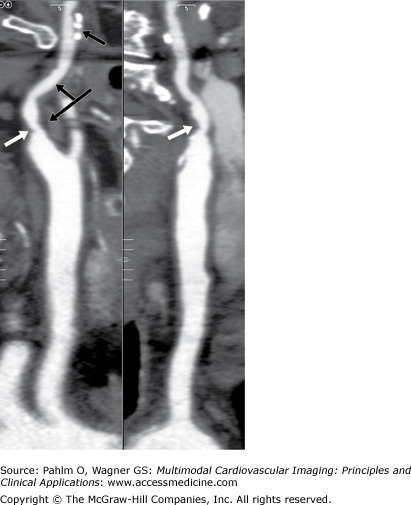
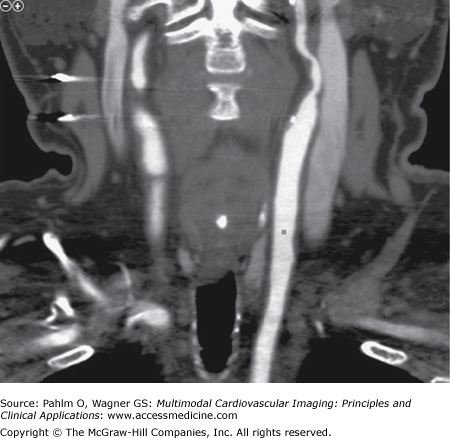
Carotid DUS shows a “possible complete occlusion” of the left common carotid artery and “mild” disease within the right carotid system. The patient is referred to cardiology, and a CTA of the neck is ordered for further clarification. See Figs. 23–1, 23–2, 23–3.
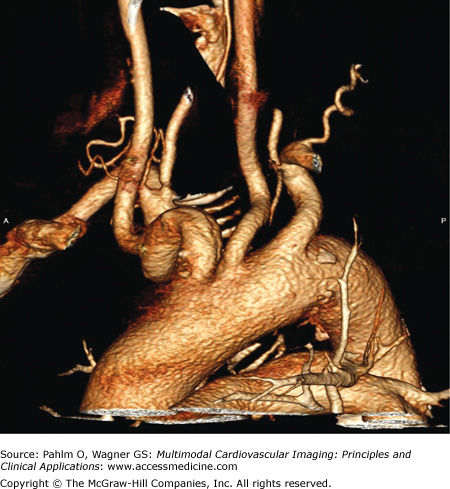
Figure 23–2.
Volume-rendered (VR) view of the aortic arch. The classification of the arch is important when exploring an invasive strategy. This is a class II arch with the innominate, left carotid, and subclavian arteries slightly below the apex of the arch. The vertical distance from the origin of the innominate artery to the top of the arch determines the arch type. Type 1 is less than one time the diameter of the common carotid artery (CCA), type 2 is one to two times the diameter of the CCA, and type 3 is greater than two times the diameter of the CCA. Also note the tortuosity for the innominate artery.
Stroke is the third leading cause of death in the United States, with an estimated 610,000 new strokes and 185,000 recurrent strokes diagnosed each year.1,2 Strokes are costly to diagnose and treat, with cost estimates of $73 billion for 2010.1 An estimated 15% to 30% of all strokes are related to atherosclerotic disease within the carotid bifurcation.3 The atherosclerotic process is postulated to be similar to that of coronary atherosclerotic disease involving areas of low shear stress, inflammation, lipid accumulation, and activation of enzymatic processes.4 The two main mechanisms of transient ischemic attack (TIA) or stroke caused by atherosclerotic disease within the carotid system are embolization and reduced blood flow in the setting of in-adequate collateral circulation or impaired vasoreactivity.5
The patient is a 68-year-old man with several risk factors for stroke and carotid disease who appears to be suffering TIAs in the form of stuttering focal right hand paresthesias. The neurologic symptoms that suggest TIA or stroke related to carotid artery disease are focal and typically involve the ipsilateral middle cerebral artery. TIAs are defined as transient episodes of neurologic dysfunction that are caused by a disruption of blood flow to the central nervous system that does not cause permanent injury and resolve within 24 hours. In the United States, the incidence of TIA is estimated at 200,000 to 500,000, and the prevalence is approximately 2.3%, or close to 5,000,000 people.6 At least 15% of all strokes are preceded by a TIA, underscoring the importance of patients and clinicians pursuing an aggressive evaluation and management strategy.7 The risk of stroke is highest in the 30-day period following a TIA, with the North American Symptomatic Carotid Endarterectomy Trial (NASCET) demonstrating a 20% stroke risk at 90 days, and other studies showing a 1-year mortality of 25%.8,9 These studies demonstrate the need for relatively urgent imaging for risk stratification in patients who have recently experienced TIAs. Factors that predict an upcoming stroke in patients experiencing a TIA are age >60 years, diabetes mellitus, focal weakness or speech impairment, and symptoms that last for longer than 10 minutes.10
TIAs related to carotid disease are usually caused by embolization of debris from the carotid arch or can be caused by low cerebral perfusion as a result of a significant carotid stenosis in the setting of inadequate collateral circulation and impaired vasoreactivity. Clinically, the low flow states usually present with brief symptomatic episodes, whereas embolic phenomena generally result in more prolonged symptoms. The risk of low flow TIAs resulting from total occlusion of the internal carotid is generally greatest at the time of occlusion but can also occur weeks to months later due to thrombotic embolization of the occlusive material, resulting in a delayed stroke.
The risk of stroke caused by carotid plaque is directly correlated with the degree of carotid stenosis.11 Carotid disease is progressive; patients with high-grade stenosis should be followed up every 6 months, and patients with moderate stenosis should be followed up annually. Plaque characteristics such as ulceration, degree of fibrous cap formation, and inflammatory factors also play a role. Active thrombotic plaque is usually found in patients who have suffered a stroke, whereas plaque from patients suffering TIAs or who are asymptomatic are much less likely to show active thrombus.12 Another important finding was that active thrombus was still found on plaques that were removed almost a year after symptom onset.
The patient’s symptom of stuttering right arm paresthesias is a common and typical presentation of systemic hypotension leading to reduced cerebral perfusion in a patient with poor collateral circulation, although this could also have been a result of embolic phenomena. The patient was noted to have bilateral carotid bruits. Although assessment of bruits is recommended, the presence or absence of a bruit is a poor predictor of future stroke or significant carotid disease in asymptomatic patients.13 Bruits represent a hemodynamically significant lesion 30% of the time; however, in patients with hemodynamically significant lesions, a bruit was detected in only 50% of patients.14
The use of invasive arteriography and digital subtraction angiography is considered the gold standard when evaluating carotid artery disease. The incidence of stroke during the procedure is greater than 1% in most series, and there are additional morbidities such as access site complications, contrast nephropathy, and the exposure of patients and operators to radiation.15 Given these limitations, noninvasive imaging has taken on a larger role for screening and preoperative assessment of carotid disease. In this case, the initial imaging modality chosen was carotid DUS. The assessment is performed by evaluating peak systolic and end-diastolic velocities proximal and distal to the stenosis. The limitation of DUS is that its effectiveness is operator and patient dependent, with some studies estimating a misclassification rate as high as 25%.16 In general, it is felt that DUS is similar to MR and CT imaging in detecting complete occlusions but less effective in evaluating nonocclusive lesions.16 For stenoses in the 70% to 99% range, the sensitivity and specificity for DUS are 83% and 89%, respectively.17 The limitation of DUS in differentiating between complete and severe occlusions is important because procedural treatment is not indicated in complete occlusions. Given this, alternative confirmatory imaging modalities such as MR angiography (MRA) and CTA play a large role in patients who are likely to undergo carotid surgical or intravascular intervention. In the case presented, a CTA was chosen. This modality has a sensitivity of 80% to 95% and a specificity of 92% to 99% for lesions in the 70% to 99% range, with greater sensitivity and specificity noted in studies using multirow scanners.
In summary, CTA offers an excellent platform when carotid duplex is ambiguous, permitting visualization of aortic arch or high bifurcation pathology, reliable differentiation of total and subtotal occlusion, and assessment of ostial and tandem stenosis. Screening of the general population with imaging studies is not recommended but may be indicated in some patients with a higher prevalence of disease.
In our case, the CT showed the left internal carotid artery (ICA) to be severely stenotic but not occluded. The DUS showed a “possible complete occlusion,” which is an absolute contraindication for surgical or interventional treatment approaches. Given the findings of CTA, our patient was referred for carotid endarterectomy (CEA).
- Carotid artery atherosclerotic disease is a major cause of TIA and stroke in symptomatic and asymptomatic individuals.
- The risk of stroke is correlated with the degree of carotid stenosis.
- TIAs are a warning sign of future stroke; therefore, imaging and evaluation should take place promptly after a patient experiences symptoms.
- DUS is an effective screening test. Limitations include high variability, operator dependence, and difficulty in distinguishing whether there is a complete occlusion or severe stenosis.
- Screening for carotid artery disease is not recommended for the general public but can be helpful in risk stratifying high-risk individuals.
- CTA is an excellent modality to evaluate the carotid arteries that is both highly sensitive and specific.
Case 2: Carotid Artery Disease
The patient is a 67-year-old man with a history of coronary artery disease and multivessel percutaneous coronary intervention several years ago. He is sent for a DUS of his carotid arteries following the complaint of visual changes in his left eye, which shows a left carotid artery stenosis of 80% to 99%.
The patient undergoes an endarterectomy of the left ICA, but his postoperative course is complicated by right-sided weakness. He returns to the operating department, where a large dissection is noted along the length of the left ICA. Attempts to repair are unsuccessful, and the patient is started on Plavix, aspirin, and Coumadin. He is transferred to the catheterization laboratory for urgent angiography, which verifies the dissection.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree