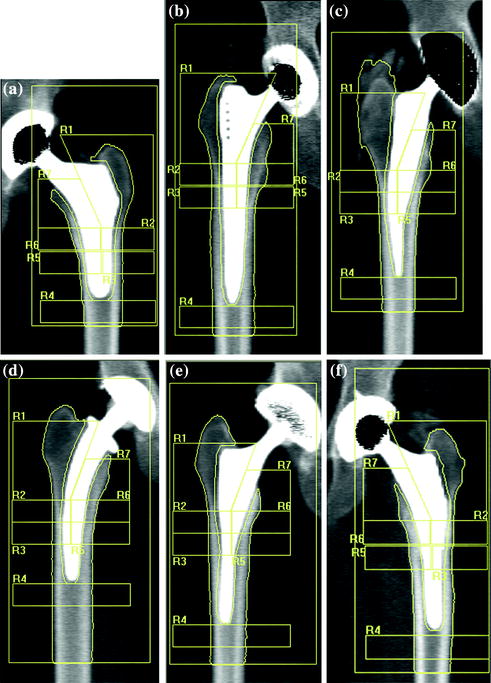
Fig. 8.2
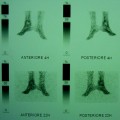
Periprosthetic DXA was used to compare bone mass after uncemented THA of a custom-made stemless design (a) with five groups of conventional cementless implants: Alloclassic (b), Mayo (c), CFP (d), IPS (e) and ABG (f). The adaptive bone changes of the proximal femur 3 years after implantation were evaluated. To allow the comparative analysis of prosthesis with different length of the stem, ROI 3 and 5 are placed more proximally with respect to standard Gruen analysis protocol [33]
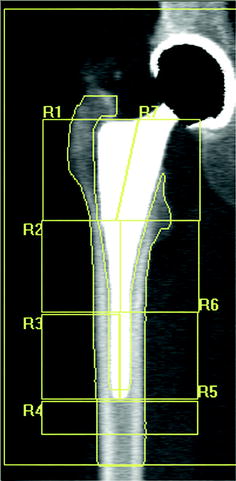
Fig. 8.3
Periprosthetic DXA: example of 7-ROI protocol of analysis according to Gruen zone
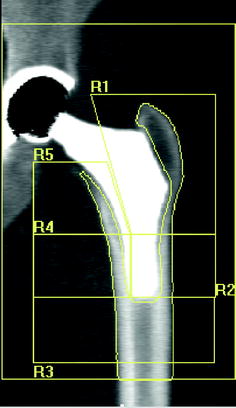
Fig. 8.4
Type 1 custom-made femoral implant featuring an extremely short distal stem. DXA images of the proximal femoral periprosthetic analysis with 5 regions of interest: R1–R5, [20]
Accuracy. In bone densitometry, accuracy describes the degree to which the measurement of bone density reflects the true bone density. In other words, if the bone in question was removed from the body, measured, and then ashed and assayed, the true bone density could be determined. The accuracy error is usually described as %CV that describes the proportion by which the individual measurements vary from the mean value as a percentage that is synonymous with “true BMD”. Therefore, lower values of %CV are better than higher because of the %CV describing the variability of the measurement about the true BMD. Different factors may affect the accuracy. It was reported that bone cement infiltration into bone and the cement mantle around the prosthesis may affect accuracy because they determine an artificial increase in BMD [3]. A study on implanted cadaver femora reported [24] that positioning of patients is essential to obtain reliable results. Rotation of the femur about its longitudinal axis altered the BMD measurement. The largest variations with rotation were in region 7, the calcar and lesser trochanter: 15° internal rotation caused a 24 % difference compared with neutral rotation. This is important as it is in this area where marked bone remodeling and resorption occur after joint replacement [13].
Precision. Precision is the ability to reproduce the measurement when it is performed under identical conditions when there has been no real biological change in the patient. Monitoring periprosthetic bone after stem implantation provides insight into the pattern of stress redistributions that occur after implant insertion [3, 4], so the precision error of bone density testing assumes great importance when the technique is used to follow changes in bone density over time in this specific context. Like accuracy, precision is usually described as %CV. Again, the smaller the %CV is, the better the precision of the technique. Accuracy is far less important than precision. This is because it is the magnitude of the difference between measurements that is of interest.
Different factors may affect the DXA periprosthetic scan reproducibility. The precision relies on the quality of the scanner, the quality of the analysis software [25], mode of scan analysis in the case of cemented prosthesis [21], position of scanned limb [22], and the homogeneity of positioning of the patients at follow-up investigations [23]. The in vivo precision error of periprosthetic BMD measurement ranges from 1 % to 7.5 % depending on the ROI and type of the prosthetic stem [17, 24, 26]. Cohen et al. [24], in a study designed to evaluate the DXA accuracy, reported that the most significant factor affecting reproducibility was rotation of the femur. They found a CV variation of 2, 2.7, and 1 % using a hydroxyapatite phantom, an anthropomorphic phantom specimen, and in repeated measurements on implanted cadaver femora, respectively. In patients, the precision error was 1.1–4.5, depending on the ROI and rotation of the femur particularly in the region of calcar (ROI 7 according to Gruen analysis).
8.3 Periprosthetic Hip
The preoperative application of DXA, in THA to prospectively evaluate implant primary stability, is started from the observation that the efficiency of a prosthesis stem and the type of fixation are dependent on the degree of mineralization of the bone in which the prosthesis is implanted [27]. In case of poorly mineralized bone, the cemented prostheses are more suitable while in well mineralized bone with a potencially higher long-term mechanical quality, uncemented prostheses are better suited. DXA has been extensively used to evaluate the bone remodeling pattern associated with uncemented or cemented femoral stem implants. Bone cement alone or mixed with radiopaque substances such are barium or zirconium is the cause of artefacts in BMD measurements. Uncertainty remains as to whether mixed or alone cement should be included or excluded from analysis of ROIs when measuring BMD around cemented femoral implants. This had led various authors to study mainly prostheses fixed without cement [1, 2, 8, 12, 13, 17, 18].
Wilkinson et al. [21], in a study aimed at determining the effect of bone cement on the measurement of BMD in femoral ROIs after THA, reported that manual exclusion of cement from femoral ROIs increased the net CV from 1.6 to 3.6 % and decreased the measured BMD by 20 %. They concluded that manual removal of cement may be of use in population studies but of limited value in the monitoring of individuals. The main reason for this poor precision lies in the difficulty of consistently removing the same amount of cement from baseline and subsequent analyses. Venesmaa et al. [28] in a prospective 5-year study were likewise unable to distinguish cement from bone. However, it can be assumed that the density of the cement mantle does not change with time [16]. Therefore, to estimate long-term changes in periprosthetic bone density after cemented THA, BMD should be measured in the immediate postoperative period because all the BMD changes found during follow-up reflect the periprosthetic BMD measured baseline.
Uncemented prostheses, ensure high primary stability that on one hand reduces the risk of stress-shielding (loosening of the prosthesis) and on the other promotes the progressive bone integration between bone and prosthesis for direct adhesion. The evaluation of preoperative BMD makes it possible to obtain precise information about the mechanical quality of bone in the individual patient [28]. Patients with low preoperative BMD risk lose more bone near the prosthesis. Bone loss may make revision surgery more complicated or predispose to periprosthetic fractures. It was also shown that results obtained from “standard femoral DXA”, can be used to provide the surgeon with useful data about the mechanical characteristics of certain areas of the femur involved in the fixing and support of the prosthesis, in particular the subtrochanteric region of the lesser trochanter that corresponds to the diaphyseal portion, bearing the maximum stress after insertion of a stem [29].
One of the most interesting and widely studied applications of DXA in orthopaedics is the evaluation of the changes of periprosthetic bone mass during the follow-up (secondary stability), also in relation to the design of the prosthesis stem. This application is started following the observation, in some patients of a marked demineralization in the proximal regions of the femur after implantation of a cemented or uncemented prosthesis that influenced the mechanical stability of the implant [30, 31].
In long-lasting implants, the persistence of the phenomenon of stress-shielding and bone ageing, which is manifested by the endosteal enlargement and reduction in cortical thickness, are also additive potential causes of failure of the anchorage between bone and prosthesis. The survival of an implant, therefore, depends on a number of factors, such as the mechanical stability achieved, the bone integration with the bone that hosts it, the stem type used, the surgical procedures adopted, and the bone quality [32].
DXA, along with other standard diagnostic methods (conventional radiography, bone scintigraphy, CT, and RM), has contributed substantially to being able to respond to questions relevant to the evaluation of the survival of the prosthesis. The measure of bone stock, from the first studies performed with DXA, has indirectly resulted in a measure for the redistribution of the mechanical loading created from a particular prosthetic design and the consequent biologic response [2, 11]. Bone response seems to differ between different stem designs and type of fixation. Thus, bone densitometry has an important role assessing new prosthesis designs. Over the years, different protocols of analysis have been proposed in order to evaluate the bone redistribution around the implant with regard to specific stem designs obtaining satisfactory results about accuracy and precision [2, 10, 11, 16, 17] and clinical outcome.
Figure 8.1 shows the main application models proposed by various authors in the evaluation of bone changes after hip arthroplasty using DXA technique. In a cross-sectional multicenter clinical study [33], we have used a modified Gruen protocol of analysis to evaluate BMD changes in the periprosthetic bone of five femoral uncemented conventional implants compared with a custom-made stemless implant proposed by Santori et al. [34, 35]. The study was aimed at evaluating the effect on bone remodeling of the proximal loading device with metaphyseal geometry (lateral flare). In order to compare a shorter stem implant to longest stems, six ROIs were placed more proximally and one under the tip (Fig. 8.2) with respect to the standard Gruen analysis protocol (Fig. 8.3). The short-term precision error was 1.8 %. The precision varied from 1.0 to 2.9 %, depending on the ROI. The short implant showed better strain distribution, resulting in a more favorable pattern of bone remodeling in the ROIs known to be at high risk of bone loss (calcar and greater trochanter). A similar finding was reported [20] when a short implant was compared to ultra-short custom-made femoral stem (Figs. 8.4, 8.5), and to another short-stem design with the same rationale [36]. In this study, a five-ROI protocol of analysis was proposed to test the flexibility of DXA in adapting the protocol of periprosthetic analysis to the specific requirements of new implant designs and its sensibility in the evaluation of the biological response of bone to changes in implant shape. The reproducibility was consistent with the literature [12, 25], ranging from 2.8 to 3.4 % in the implanted hip and from 2.5 to 3.7 % in the contralateral unoperated hip. Recently, Lazarinis [37] in a prospective cohort study on the short collum femoris-preserving stem showed that substantial loss in proximal periprosthetic BMD cannot be prevented by the use of a novel type of short, curved stem, and forces appear to be transmitted distally. They reported a precision error between 1.1 and 5.7 % for the seven ROIs that were studied. However, the DXA images allow us to observe that the dividing line between zones 6 and 7 was not correctly placed in the mid-line of the lesser trochanter. Furthermore, the ROI 6 (that entirely includes the lesser trochanter) has been positioned too close to the bone tissue. These factors may be the sources of variability in the assessment of bone loss. These methodological considerations were allowed by the fact that the authors showed their protocol of analysis as a DXA printout image. However, despite a great variability of the periprosthetic protocols of analysis proposed in the literature, most of the published investigations reported instead of the “real bone densitometry images” [13, 20, 21, 33, 35, 36] a “schematic drawing” [2, 12, 24, 38–40] of their DXA analysis. This could generate some remarkable differences with respect to the actual analysis performed using the DXA metal/removal or orthopaedic software, thus not allowing the readers to both evaluate the reported results and reproduce the described protocol.
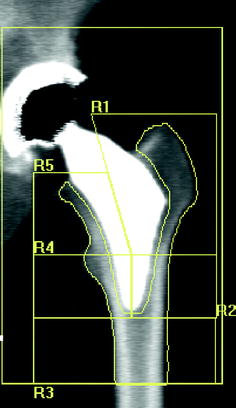

Fig. 8.5
Type 2 custom-made femoral implant featuring an almost complete absence of the stem. DXA images of the proximal femoral periprosthetic analysis with 5 regions of interest: R1–R5, [20]
8.4 Periprosthetic Knee
DXA periprosthetic analysis software was applied to total knee artroplasty (THA) less than in hip prostheses. DXA was mainly used for the assessment of bone remodeling of the tibial plate and/or of the femoral condyles after TKA. The first report of local bone mass measurements after TKA was by Seitz et al. [41




Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree