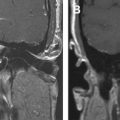
The petrous apex is the most medial portion of the temporal bone that cannot be directly examined on clinical examination. The referring physician completely relies on imaging and radiologic interpretation. Unfortunately, the petrous apex displays anatomic variations such as asymmetric pneumatization that might be mistaken for underlying lesions. The location of the petrous apex also typically precludes safe percutaneous biopsy. Knowledge of the petrous apex anatomy, normal anatomic variations, and their differentiating features from pathologic entities is critical for accurate interpretation.
The petrous apex is the most medial portion of the temporal bone that cannot be directly examined on clinical examination. The referring physician completely relies on imaging and radiologic interpretation. Unfortunately, the petrous apex displays anatomic variations such as asymmetric pneumatization that might be mistaken for underlying lesions. The location of the petrous apex also typically precludes safe percutaneous biopsy. Knowledge of the petrous apex anatomy, normal anatomic variations, and their differentiating features from pathologic entities is critical for accurate interpretation.
Anatomy
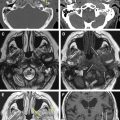
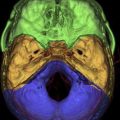
The petrous apex represents the pyramidal shaped medial portion of the temporal bone located between the inner ear structures laterally, petrosphenoid fissure anteriorly, and petro-occipital fissure medially ( Fig. 1 ). It is obliquely positioned within the skull base, with the apex anteromedially located and the base posterolaterally located. Its anterosuperior portion forms the floor of the middle cranial fossa while the posterosuperior portion constitutes the anterior wall of the posterior cranial fossa. The petrous apex is subdivided by the internal auditory canal into an anterior and a posterior portion ( Fig. 2 ). The larger anterior portion of the petrous apex typically contains bone marrow, whereas the smaller posterior portion is denser in appearance because it derives from the otic capsule (see Fig. 2 ). In 35% of patients, the petrous apex is pneumatized by infralabyrinthine, anterior, superior, posteriormedial, or subarcuate tracts that directly communicate with the mastoid or middle ear cleft ( Fig. 3 ). These tracts provide direct “pathways” for diseases to spread from the mastoid or middle ear cavity to the petrous apex.
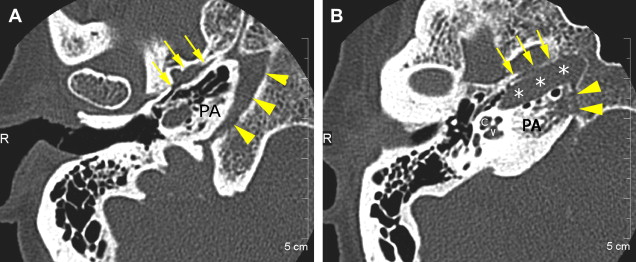
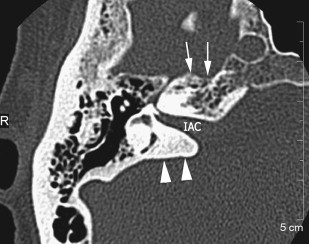
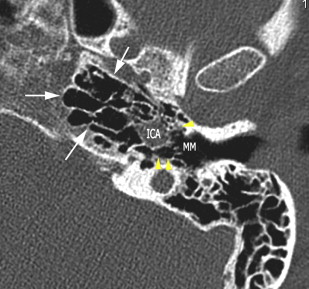
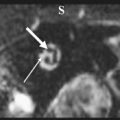
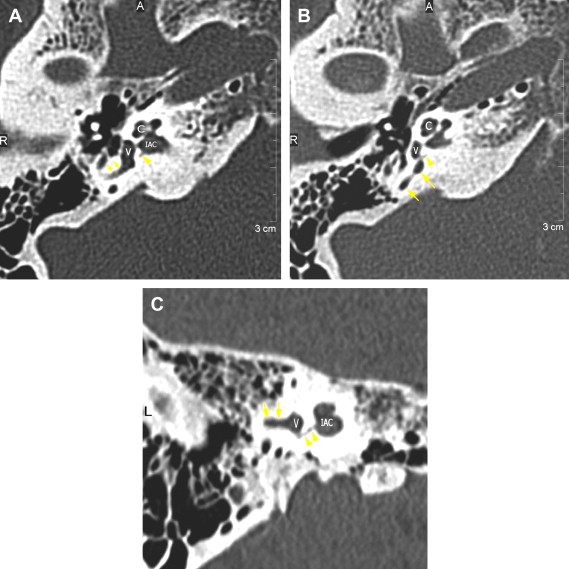
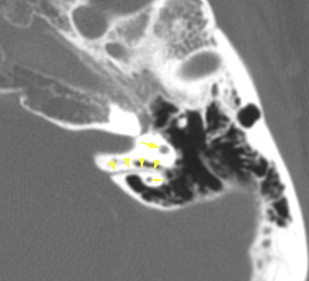
The petrous apex houses a few important vascular and neuronal channels. The petrous carotid and the internal auditory canal are the largest channels, whereas the Dorello’s, singular, and arcuate canals are markedly smaller and not always visualized. The internal auditory canal is located within the midsubstance of the petrous apex. It runs in coronal plane in relations ship to the skull but in an angle of approximately 45° in relation to the long axis of the petrous bone. It houses the vestibulocochlear and facial nerves (cranial nerves VIII and VII, respectively). Within the internal auditory canal, the facial nerve is located in the anterior superior portion. The cochlear nerve runs in the anterior inferior portion of the internal auditory canal, whereas the superior and the inferior divisions of the vestibular nerve course in the superior posterior and inferior posterior portions of the internal auditory canal, respectively. Within the lateral portion of the internal auditory canal, all four nerve components can be seen on volumetric, heavily T2-weighted images, whereas only two nerves are identified in the medial portion ( Fig. 4 ) because the vestibulocochlear nerve separates first in the mid portion of the internal auditory canal into its three components (cochlear, superior vestibular, and inferior vestibular divisions). The inferior vestibular nerve also divides within the lateral half of the internal auditory canal into the saccular nerve and the singular nerve, with the singular nerve leaving the internal auditory canal through a small posterior inferior located channel called the singular canal ( Fig. 5 ). The singular canal ends at the junction of the ampulla of the semicircular canal and the vestibule.

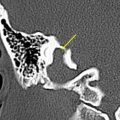
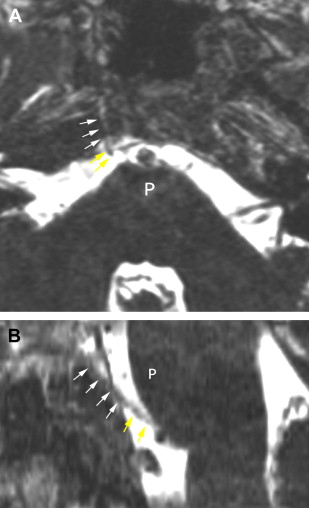
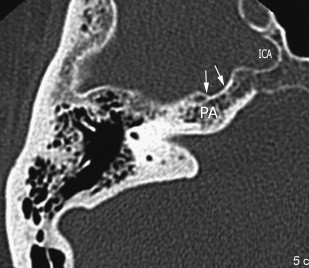
The petrous carotid canal within the anterior portion of the petrous apex contains the horizontal portion of the internal carotid artery (see Fig. 1 B). The foramen lacerum is the fibrocartilagenous, medial extension of the bony floor of the petrous carotid canal that is located between the petrous apex, the basiocciput, and the basisphenoid bone. The Dorello’s canal extends through the medial portion of the petrous apex and contains the abducens nerve (cranial nerve VI) ( Fig. 6 ). The arcuate canal courses between the crura of the superior semicircular canal within the superior portion of the petrous apex and holds the arcuate artery ( Fig. 7 ). A marked depression is seen in the anterior superior aspect of the petrous apex that is caused by the Meckel’s cave ( Fig. 8 ). The Meckel’s cave contains the gasserian ganglion and the rootlets of the trigeminal nerve.
Symptomatology
Patients with petrous apex lesions are typically asymptomatic or present with nonspecific symptoms, such as headache, retro-orbital pain, or ear pain. When the lesions become larger or are located close to the cranial nerves in or in close proximity to the petrous apex, patients may complain of sensorineural hearing loss, vertigo, double vision, facial pain, or facial weakness.
Symptomatology
Patients with petrous apex lesions are typically asymptomatic or present with nonspecific symptoms, such as headache, retro-orbital pain, or ear pain. When the lesions become larger or are located close to the cranial nerves in or in close proximity to the petrous apex, patients may complain of sensorineural hearing loss, vertigo, double vision, facial pain, or facial weakness.
Normal anatomic variations and pathologic entities of the petrous apex
Primary petrous apex abnormalities can be divided based on origin within the petrous apex, petrous apex size, and lesion appearance into four major categories: lesions related to neurovascular channels, lesions without petrous apex enlargement, lesions with petrous apex enlargement and nonaggressive appearance, and lesions with aggressive appearance. Although some of these entities might show no significant enlargement of the petrous apex or nonaggressive features in the early stages of the disease, such categorization facilitates establishment of a reasonable list of differential diagnostic considerations. This approach is used in the subsequent discussion of petrous apex abnormalities.
Primary Petrous Apex Lesions Related to Neurovascular Channels
Narrow internal auditory canal syndrome
Narrow internal auditory canal syndrome is a rare malformation of the temporal bone that manifests with congenital sensorineural hearing loss. It is defined as internal auditory canal diameter of less than 2 mm and is caused by absence of the vestibulocochlear nerve ( Fig. 9 ). Typically, this anomaly is unilateral and associated with a duplication of the internal auditory canal, with one canal containing the facial nerve and the second canal being empty (see Fig. 9 D).
Schwannoma
In the petrous apex region, schwannomas may arise from the fifth, seventh, and eighth cranial nerves, with the vestibular division of the eighth cranial nerve being most commonly involved and accounting for 5% to 10% of all intracranial tumors. Unilateral involvement is most commonly observed, with bilateral involvement that occurs in association with neurofibromatosis type II seen in less than 5% of patients. Acoustic schwannomas typically originate around the vestibular ganglion near the fundus of the internal auditory canal. Sensorineural hearing loss is the most common symptom and is caused by compression of the cochlear division of the eighth cranial nerve with progressive increase in size of the tumor. Surprisingly, dizziness is only rarely observed. These tumors are known to grow slowly—approximately 1 to 2 mm per year.
Imaging shows a well-defined mass filling up the internal auditory canal with progressive expansion of the bony canal and extension into the cerebropontine angle over time that may result in mass effect on the cerebropontine angle. The appearance of schwannomas has been described as an ice cream cone, with the “cone” situated within the internal auditory canal and the “ice cream” in the cerebropontine angle ( Fig. 10 ). They show low signal intensity on T1-weighted and high signal intensity on T2-weighted images in relation to the adjacent brain parenchyma. Typically, marked, homogenous enhancement is seen after contrast administration (see Fig. 10 ). Occasionally, acoustic schwannomas might extend laterally into the labyrinth or even into the middle ear cavity via the round or oval window requiring more extensive surgical resection. Isolated involvement of the labyrinthine structures of the vestibule, semicircular canals, or cochlea also has been described ( Fig. 11 ).
Facial nerve (seventh cranial nerve) schwannomas are rare. They usually originate from the geniculate ganglion and progress proximally or distally from it. On MR imaging, they show the same signal characteristics as the acoustic schwannomas and may be indistinguishable from them when the facial nerve schwannoma is isolated to the internal auditory canal ( Fig. 12 ). Trigeminal nerve (fifth cranial nerve) schwannomas are also rare and usually arise within the Meckel’s cave or from the cisternal segment of the nerve ( Fig. 13 ). As with facial nerve schwannomas, they grow ante- and retrograde along the nerve and its branches, causing expansion of the transpassing neural foramina and superior indentation on the petrous apex. In contrast to the acoustic and facial nerve schwannomas, the trigeminal nerve schwannomas have a markedly higher propensity for containing cystic components (see Fig. 13 ).
Aberrant internal carotid artery malformation
Aberrant internal carotid artery malformation ( Fig. 14 ) is a rare vascular congenital malformation of the petrous apex that is caused by regression of the cervical internal carotid artery during embryogenesis. On imaging, it can be recognized by markedly more posterior lateral extension of the petrous carotid canal that is contiguous with an enlarged inferior tympanic canaliculus within the middle ear cavity. On axial images, the aberrant internal carotid artery is usually easily recognized, whereas on coronal images it might be mistaken for a glomus tumor. Such a misinterpretation may become disastrous because biopsy or removal of an aberrant carotid artery may result in uncontrollable bleeding or major cerebral infarction from vascular injury. Vigilant evaluation of the course and intactness of the internal carotid artery canal are essential (see Fig. 14 A; see Fig. 1 B). In uncertain cases, MR angiography or CT angiography might be helpful.
Primary Petrous Apex Lesions without Petrous Apex Enlargement
Asymmetric pneumatization
In most patients, the petrous apex is nonpneumatized in nature. Even if pneumatization occurs, it is typically symmetric in appearance and not of diagnostic concern. Only when the pneumatization is asymmetric is the nonpneumatized petrous apex typically misinterpreted as a petrous apex lesion such as cholesteatoma, petrous apicitis, or cholesterol granuloma. Such an incorrect interpretation rarely occurs with CT, in which the lack of mass effect and the preservation of the cortex or bony trabecula are easily visualized. In contrast, MR imaging is more confusing because the nonpneumatized petrous apex shows variable signal intensity depending on a patient’s age. In younger patients, it is of intermediate signal intensity on all sequences and is unlikely to be mistaken for cholesteatoma or fluid- or pus-filled petrous apex, which typically shows high signal intensity on T2-weighted images. In contrast, the high T1 signal intensity of the fatty bone marrow within the nonpneumatized petrous apex in older adults may be mistaken for cholesterol granuloma. Lack of mass effect and close observation of the signal intensity usually lead to the correct diagnosis. The fatty bone marrow shows suppression of the T1 hyperintensity on fat suppressed sequences ( Fig. 15 ). Cholesterol granuloma often shows even higher T1 signal intensity than the surrounding bone marrow or even subcutaneous adipose tissues.