CHAPTER 26 Pharmacologic Stress Agents
VASODILATOR AGENTS
Coronary Blood Flow
As heart muscle contracts during systole, the myocardium impedes its own blood supply because most CBF occurs during diastole. This pulsatile flow is conducted through a highly curved, branching vascular system composed of larger epicardial vessels and smaller intramural vessels. While epicardial coronary arteries serve as conductance vessels, intramural arteries distribute and regulate blood flow. This adaptation, termed autoregulation, occurs rapidly in response to changes in myocardial oxygen demands, primarily through functional hyperemia.1 This ability of the coronary tree to alter its resistance and increase CBF in response to myocardial metabolic demands is maintained by a balance between vasoconstrictors, such as endothelin, and vasodilators, such as nitric oxide. Acting in smaller arteries and arterioles, these vasoactive factors are integral in allowing rapid, sustained adaptation of CBF based on the requirements of the myocardium.
Coronary Flow Reserve
As atherosclerosis develops in epicardial coronary arteries, the resulting stenosis produces resistance to blood flow. The physiologic effect of a coronary stenosis depends on the degree to which the resistance to flow can be compensated by dilation of the microcirculation distal to the stenosis. This mechanism, regulated by vasoactive factors, maintains resting CBF at a constant level despite the presence of a growing burden of atherosclerosis. When an epicardial coronary constriction exceeds 80% to 90% of the normal segment diameter, the distal vasodilatory mechanisms are unable to compensate, and resting CBF cannot provide adequate oxygen to meet the resting needs of the myocardium.1,2 When a stenotic vessel is exposed to a vasodilator (e.g., adenosine or dipyridamole) intended to induce hyperemic (or maximal) flow, the relative increase in flow decreases proportionately when a stenosis exceeds 50%, and is generally abolished at a 90% occlusion (Fig. 26-1).
Coronary Blood Flow and Radiotracer Uptake
Vasodilators can increase CBF five times normal levels. This large increase in CBF occurs in myocardium supplied by normal coronary arteries, in contrast to the lack of an increase to myocardium supplied by stenosed arteries as discussed previously; this forms the basis for vasodilator perfusion imaging (Fig. 26-2). If the myocardial uptake of radiotracers such as thallium 201 and Tc 99m were purely linear and based on CBF, a high level of image contrast would theoretically exist between myocardium supplied by normal arteries (i.e., without stenosis) and arteries with hemodynamically significant stenoses. In reality, the extraction fraction of most tracers decreases when CBF increases beyond 2.5 times baseline values. The practical effect of this “roll-off” in the uptake-flow relationship is a physiologic constraint in detecting mild-to-moderate stenoses. The flow increase in response to vasodilators in vessels with a mild-to-moderate stenosis may still be two to three times normal because of coronary flow reserve. This prevents differentiation with a normal coronary artery owing to radiotracer roll-off at a fixed level of hyperemic flow because the normal and mildly stenotic arteries would have identical tracer uptake. Generally with the more common radiotracers, it is difficult to identify noninvasively stenoses that are less than 50% because of the preservation of CBF in the resting and hyperemic states through natural vascular response mechanisms.
Adenosine
Adenosine is a small heterocyclic compound that is endogenously produced in variable amounts as part of a normal cellular metabolism and during ischemia. Through adenosine receptors, it serves as a potent coronary vasodilator (adenosine A2 receptors) and inhibitor of the sinoatrial and atrioventricular (AV) nodes (adenosine A1 receptors).3 Adenosine is produced intracellularly, but interacts on the adenosine receptors on the outer cell membrane (Fig. 26-3). Theophylline and caffeine act at these receptors as competitive blockers, reducing the effects of adenosine.
Clinically, adenosine is infused intravenously and has a very short half-life of less than 2 seconds. As a result, the time-to-peak effect occurs in the first minute of infusion. Adenosine is given typically as a continuous infusion of 140 µg/kg/min for 4 to 6 minutes, with radiotracer injection halfway into the infusion. To avoid an unintended rapid bolus of residual adenosine, radionuclide dose injection and flushing and adenosine infusion should be done through separate intravenous lines or through the use of a dual port.3 Hemodynamically, adenosine causes a modest decrease in systolic blood pressure and increase in heart rate, in total leading to an increase in cardiac output and mild increase in pulmonary capillary wedge pressure.
Side effects are common with adenosine infusion, occurring in more than 75% of patients (Table 26-1). These side effects are transient, usually resolving within 1 to 2 minutes of stopping the infusion, and rarely require reversal with aminophylline. Most side effects are related to stimulation of the adenosine A1 and A3 receptors. Chest pain is common, although it is not specific for the presence of coronary artery disease, occurring in patients with no coronary artery disease at the same frequency as in patients with documented coronary artery disease. Although stimulation of the adenosine A3 receptors can theoretically lead to bronchospasm, most dyspnea occurring with adenosine infusion is likely related to hyperventilation. Caution should be used in patients with a history of asthma, severe chronic obstructive pulmonary disease (COPD), or active wheezing because of the potential for bronchospasm. Changes in AV conduction usually result in transient first-degree or second-degree AV block. Most bradyarrhythmias are transient and resolve despite continued adenosine infusion.
Dipyridamole
Dipyridamole is a pyrimidine base that induces vasodilation by elevating blood and interstitial levels of adenosine. By blocking reuptake of adenosine into cells, dipyridamole increases extracellular levels of adenosine, allowing interaction with adenosine A2 receptors on the outer cell membrane. Activation of the adenosine A2 receptor leads to a complex series of events mediated by G proteins and leading to a decrease in intracellular calcium uptake, culminating in coronary vasodilation (see Fig. 26-3). Dipyridamole also exhibits antiplatelet activity, likely related to phosphodiesterase inhibition, increased adenosine triphosphate (ATP) levels, potentiation of aspirin effects, and increased adenosine.3
Clinically, dipyridamole is infused intravenously and has a biologic half-life of 88 to 136 minutes. It is given as a continuous infusion of 0.57 mg/kg over 4 minutes, with radiotracer injection 3 to 4 minutes after the dipyridamole infusion is completed. This is distinct from adenosine, where tracer injection is during vasodilator infusion (Fig. 26-4). The standard intravenous dose of dipyridamole results in a slight increase in heart rate, a decrease in systolic blood pressure, a modest increase in cardiac output, and a slight increase in pulmonary capillary wedge pressure.
Side effects occur in about 50% of patients and include chest pain, nausea, dizziness, and headache (see Table 26-1). As with adenosine, chest pain is mostly nonanginal and does not correlate with the presence of coronary artery disease. Bronchospasm is rare, but can be precipitated in patients with asthma or severe COPD. Arrhythmias, including AV block, are highly uncommon. Dipyridamole has generally been extensively studied and has an excellent safety record. Most significant side effects can be reversed with slow intravenous administration of theophylline (100 to 300 mg), which is a competitive blocker of adenosine receptors.
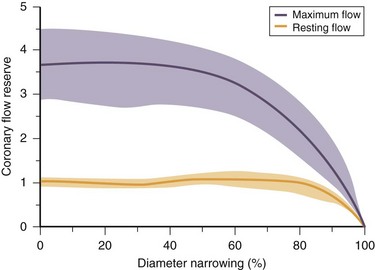
 FIGURE 26-1
FIGURE 26-1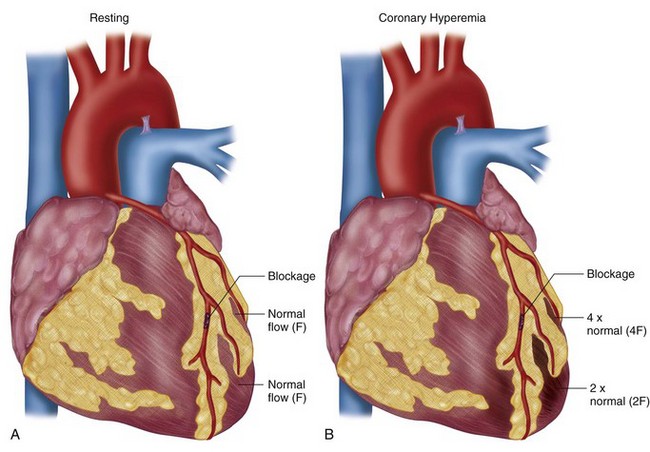
 FIGURE 26-2
FIGURE 26-2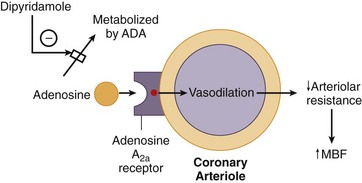
 FIGURE 26-3
FIGURE 26-3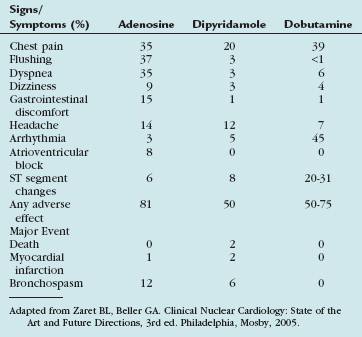
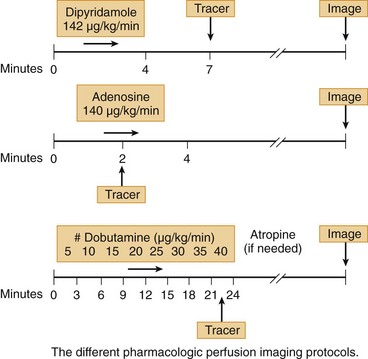
 FIGURE 26-4
FIGURE 26-4


