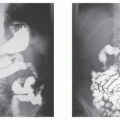
Pharmacology is the study of drug actions and drug interactions with living organisms. Drugs are chemical substances that are not required for normal maintenance of body function and produce a biologic effect in an organism. All drugs are, or can be if misused, poisons. If used correctly, they are meant to relieve human diseases and suffering.
There are three categories assigned to substances applied or administered for therapeutic purposes: drugs or medications, biologics, and alternative therapies. A drug is a chemical agent capable of producing biologic responses in the body. These responses may be desirable (therapeutic) or undesirable (adverse). After a drug is administered, it is called a medication. A biologic is an agent naturally produced in animal cells, microorganisms, or by the body itself, such as hormones, natural blood products, or vaccines. Alternative therapies include natural plant extracts, herbs, vitamins, minerals, dietary supplemental, and therapeutic techniques that may be considered unconventional, such as acupuncture.
Pharmacology and its clinical application are a scientific discipline unto itself and cannot be covered adequately without combining theoretical knowledge and clinical coursework. Drug therapy is a complex and ever-changing aspect of patient care. The radiographer neither is licensed to dispense drugs nor can enter a hospital pharmacy and select a drug. Radiographers are held legally liable if any drug taken from a dispensary results in adverse effects. If a drug error is made by the radiographer, the incident must be completely documented and fill out an institutional incident report according to the policy of the employer.
DRUG STANDARDS AND CONTROL
The federal government of the United States has standards for control of drug safety that are strictly enforced. In the early 1900s, drug enforcement laws were established to ensure the safety of drugs sold for public use. Through the years, these laws have been amended to ensure that drugs are thoroughly tested and proven safe before marketing.
Table 15-1 lists the most recent laws that regulate drug production and sale.
There are lists of drugs that must bear the legend “caution: Federal law prohibits dispensing without prescription.” They are as follows:
Drugs that must be administered parenterally
Drugs that are hypnotic or narcotic
Drugs that may cause dependence
Drugs that contain derivatives of habit-forming substances
Drugs that may be toxic if not administered under the supervision of a physician, dentist, or nurse practitioner
Drugs that are new and limited to investigational use and are not safe if indiscriminately used
Drugs that are considered safe for self-administration are called over-the-counter drugs (OTC drugs). Some drugs that must be prescribed may also be purchased as OTC drugs because they are marketed in a lesser potency when sold in this manner. OTC drugs must also be reviewed by a panel of reviewers appointed by the Food and Drug Administration (FDA) and deemed safe for self-administration. When taking a patient’s drug history as part of an initial assessment, the patient’s use of OTC drugs and alternative medications is important information because these agents may affect treatment.
The use of alternative therapies by the general public has become an accepted method of healing. Forty percent of adults in the United States today use complementary and alternative medicine (CAM). Physicians are embracing CAM and are combining it with mainstream medicine which has caused the term “integrative medicine.”
Alternative dietary and herbal supplements are not regulated by the FDA in the United States. They are regulated and classified as foods, not drugs. There is no requirement to prove the efficacy, quality, or safety of these substances. The Dietary Supplement Health and Education Act enacted in 1994 forbids the producers of these drugs from making claims that discuss disease or the cure of an ailment; however, they may claim effectiveness on body structure and/or function. This act deemed these products independent of FDA rules. In September 2010, SB 3767 was introduced that would allow the FDA to target natural health product companies to require ever stricter requirements on labeling. However, this bill was amended over and over and has been stalled in legislature.
Supplements in this classification are too numerous to include in this text, but the radiographer taking a health and medication history must include questions concerning the patient’s use of these substances because many may react in an adverse manner with other drugs.
Since 1980, the only official books or publications of drug standards in the United States are the United States Pharmacopeia (USP) and the National Formulary. The drugs listed in these books meet the high standards of quality, purity, and strength. There are several other good References for drugs that one may consult in the clinical area; however, drugs that meet the criteria of the USP can be identified by the letters USP after the drug’s official name.
DRUG CONTROL
Drug abuse continues to increase in the United States and throughout the world with each passing year. In 2013, the number of deaths that were related to drug use exceeded deaths that occurred from auto accidents. The increase is attributed to the abuse of prescription drugs. The words addiction and dependence are often used interchangeably. Addiction refers to an overwhelming feeling of physical need for a particular drug that must be met at all costs. Dependence may be a physical or psychological need for a particular drug. If the need is physical, when the drug is no longer available, the body develops physical signs of discomfort that are called withdrawal. If the need is psychological, the dependent person has no physical signs of discomfort, but continues to need the feeling that the drug produces. These abnormal needs create addiction and potential for abuse of a drug.
In an effort to restrict drug abuse, the United States enacted the Controlled Substances Act in 1971. This law was also meant to increase research and to assist persons dependent on drugs with rehabilitation. In 1973, the Drug Enforcement Administration in the Department of Justice became the nation’s only legal drug enforcement agency.
Drugs labeled as controlled substances have been categorized by the Controlled Substances Act into schedules according to numbers related to their potential for abuse. The drug schedules are listed in
Table 15-2.
DRUG SOURCES, NAMES, AND ACTIONS
Drugs come from many natural and synthetic sources. Some are produced from animal sources, such as hormonal drugs. Many come from plant sources, such as digitalis and atropine. Others are produced from microorganisms as are many antibiotics. Minerals are the source of calcium, iron, and other dietary supplements and herbal remedies. Drugs from synthetic materials are made in laboratories. Some drugs are genetically engineered and used to treat specific diseases. Learning drug names is complicated
because most drugs have several names. The following list should help to sort out the way drugs are named:
Proprietary or trade names are assigned to a drug by a particular manufacturer of the drug.
Chemical name of a drug presents its exact chemical formula of a drug and always remains the same.
Generic name of a drug is the name given to a drug before its official approval for use. It is assigned by the U.S. Adopted Name Council. This is the name used to describe the drug. There is one generic name for each drug and, because it is often used, it must be learned by all persons who administer drugs. For instance, a drug frequently administered before imaging procedures is parenteral diazepam. Diazepam is the generic name for Valium. The radiographer must be able to identify certain drugs by their trade name and their generic name.
A drug does not have the capacity to change cellular structure, but acts to either increase or decrease the rate and range of a normal or an abnormal physiologic process going on within the cells of the body. Drugs are absorbed, distributed, metabolized, and then excreted from the body. As this process takes place, the drug reaches a point at which it has its intended effect. This is called the onset of action. As it continues to be absorbed, the drug reaches its peak concentration level. This is the time during which the drug attains its maximum therapeutic response. This means that the drug is able to produce its most desired curative or remedial effect. The time during which the drug is in the body in an amount large enough to be therapeutic is called its duration of action. As the drug is excreted from the body, the concentration level subsides to a point at which there is little or no intended effect.
It is seldom possible for a health care practitioner to keep up with all new drugs that are marketed. Anyone who administers drugs must be able to obtain information concerning any drug from reliable sources before administering a drug with that is not familiar to the person administering it. Some reliable References are The American Hospital Formulary Service Drug Information, Drug Facts and Comparisons, Handbook of Nonprescription Drugs, Physician’s Desk Reference, and USP. All these References are updated yearly. The radiographer must learn where a reliable drug reference is available in the workplace and consult it as necessary.
THE ACTION OF DRUGS WITHIN THE BODY
Drugs act in different ways in the body depending on their physical makeup. All drugs must be in liquid form to be absorbed. For this reason, drugs that are administered in solid or tablet form must go through a phase called the pharmaceutic phase before they can be absorbed. This means that the solid form of the drug must be broken down into tiny particles to be dissolved into the body fluids of the gastrointestinal (GI) tract. Enteric-coated tablets do not go through the pharmaceutic phase of absorption until they reach the small intestine where they are dissolved in an alkaline media. Drugs that are administered orally in liquid form or drugs that are administered parenterally do not go through this phase.
PHARMACOKINETICS
The processes that control absorption, distribution, metabolism, and excretion of drugs by the body are called pharmacokinetics. All persons process drugs differently depending on their age, nutritional status, ethnicity, existing physical condition, immune status, state of mind, gender, weight, environmental factors, and time of day.
A drug must be absorbed and taken through the bloodstream to its intended site in order to act. The amount of drug that actually reaches the systemic circulation becomes bioavailable or reaches a state of bioavailability. Drug absorption varies from person to person and depends on the absorptive surface available. An intended drug surface that is damaged or absent alters the length of time it takes a drug to reach its intended site.
Drugs move to their site of absorption and then must penetrate the cell membrane at that site. This is accomplished by varying methods. One method is passive diffusion (passive transport), which requires no cellular energy. The drug simply moves across a cell membrane from an area of higher concentration to one of lower concentration. This is known as moving down the gradient. When the concentration equalizes on both sides of the cell membrane, the transport is complete. Lipid solubility is the most important determinant in deciding whether a drug will cross cell membranes, although water solubility is also important. Most drugs cross cell membranes by passive diffusion.
Active transport is another method of drug absorption. Active transport is necessary to move some drugs and electrolytes, such as sodium and potassium, from outside to inside a cell. This method requires energy from the cell and a carrier molecule, such as an enzyme or protein, that forms complexes with drug molecules on the membrane surface to carry them through the membrane and then leave them by disassociation. This requires energy because the drugs are moving from an area of higher concentration to one of lower concentration; or moving down the gradient. Pinocytosis is a type of active transport in which a cell engulfs a drug particle, forms a protective coat around it, and transports it across the cell membrane.
Drugs taken orally are usually absorbed in the small intestine, which has a large surface for absorption. If a portion of the small intestine has been removed or is scarred, the ability to absorb a drug is reduced.
The quantity of blood flow to absorption surfaces affects the rate at which a drug is absorbed. For instance, a drug is absorbed much more rapidly when it is administered intramuscularly in the deltoid muscle than in the gluteal muscle because the blood flow is greater in the deltoid. A person who is in severe pain or in a state of acute stress may have decreased ability to absorb a drug.
First-Pass Effect
When a drug is taken orally and swallowed into the stomach, it goes from the small intestine to the mesenteric vascular system and then to the portal vein and from there into the liver before it is transported into the systemic circulation. Because of this travel through the gastric and hepatic circulation, a portion of the drug is metabolized in route and becomes inactive. This partial metabolism of a drug before it reaches the systemic circulation is called a first-pass effect. In the cases of many drugs taken orally, this effect requires that a larger dose of a drug be administered so that a portion of the drug remains to perform its intended effect. Drugs that can be administered by sublingual, vaginal, or parenteral route avoid the first-pass effect by going directly into the systemic circulation; however, these routes of administration may be contraindicated for other reasons.
Some drugs, after absorption, are moved from the bloodstream into the liver and then through the biliary tract, where they are excreted in bile or return to the small intestine and back into the bloodstream. This action, called enterohepatic recycling, allows the drug to persist in the body for long periods.
Distribution
After a drug is absorbed into the body, it is distributed to its intended target site. The rate and extent of distribution depends on adequate blood circulation, protein binding, and the drug’s affinity for lipoid or aqueous tissues. Drugs move quickly to body organs that have a rich supply of blood, such as the heart, liver, and kidneys. They reach muscles and fatty tissues more slowly.
As a drug travels through the circulatory system, it may come into contact with plasma proteins and bind to them or remain free. If bound to plasma protein, the drug becomes inactive. Only free drugs are able to act on cells. As the free drug acts, there is a decrease in plasma drug levels, which allows a portion of the bound drug to be released and become active. The slow release of the drug allows blood levels of a drug to remain somewhat constant. The health status of the person receiving the drug and the drug itself affects drug distribution.
If two or more drugs compete for a plasma-binding site, the drug with the strongest affinity for the site acquires it. This allows a greater amount of the other drug free to act and may result in a toxic level of that drug or result in a drug-drug interaction.
Lipid-soluble drugs are stored in lipoid tissues. Highly lipid-soluble drugs remain stored in fatty tissues and are released very slowly. Drugs that are intended to penetrate the blood-brain barrier must be highly lipid soluble and bind minimally with plasma proteins to achieve their intended effect.
Because of the nonselective nature of the placenta, most drugs are able to pass that barrier and affect the developing fetus. The toxicity of drugs to the fetus during the first trimester of pregnancy makes it mandatory that the pregnant mother receives no medication without the explicit orders of her physician. Only drugs that are absolutely necessary to maintain the optimum health of the mother should be given. This is important for the radiographer to remember when caring for female patients of childbearing age.
Metabolism
The process by which the body alters the chemical structure of a drug or other foreign substance is called metabolism or biotransformation. These terms are interchangeable. Generally, this process reduces lipid solubility to render the drug ready for excretion.
Most drugs are metabolized in the liver by means of a complex chemical action involving enzymes. These enzymes act on a wide variety of compounds. In certain drugs, tissues from the plasma, kidneys, lungs, and intestinal mucosa may be involved.
Age, health status, time of day, emotional status, the presence of other drugs in the body, genetic variations, and disease states may alter the rate of drug metabolism. An altered metabolic state may allow a drug to accumulate in the body and produce an adverse reaction. Conversely, rapid metabolism of a drug may interfere
with the intended effect. Drugs administered orally are significantly metabolized by the first-pass effect through the liver, which also alters their metabolism.
Excretion
Excretion of drugs from the body takes place chiefly in the kidneys. Some drugs are excreted virtually unchanged through the kidneys, whereas others are extensively metabolized with only a small amount of the original drug remaining.
Other sites of drug excretion are through the biliary tract and into the feces or through the enterohepatic cycle and later into the kidneys. Gases and volatile liquids used for anesthesia are excreted by the lungs. Sweat and saliva are of minimal importance in drug excretion.
Some drugs or drug metabolites may cross the epithelium of the mammary glands and be excreted in breast milk. This is important if the mother is breastfeeding an infant, and a drug, particularly a narcotic drug, is transferred in high concentrations to the infant.
Half-Life
The time it takes for a 50% decrease in a drug’s presence in the body is called its half-life. It is important for a prescribing health care worker to understand a drug’s half-life in order to attain a steady-state concentration in the body. To attain this steady state, the same amount of a drug must be taken in, as is eliminated in, each 24-hour period. Drugs with a short half-life need to be administered more frequently than a drug with a prolonged half-life in order to be at maximum therapeutic level at all times.
A drug’s removal from the body is called its clearance rate. The clearance rate of a drug is an important consideration, because a drug with a slow clearance rate that is given too often may accumulate in the body and reach a toxic level. Contrast media, the drugs most used by radiographers and other imaging professionals, have a very fast distribution through the body. Because they are not metabolized as other drugs are, the contrast is excreted through the kidneys in about 24 hours.
PHARMACODYNAMICS
Pharmacodynamics is the study of the method or mechanism of drug action on living tissues or the response of tissues to chemical agents at various sites in the body. Drugs may alter physiologic effects in the body in the following ways:
1. By altering blood pressure
2. By altering heart rate
3. By altering urinary output
4. By altering function of the central or peripheral nervous system
5. By altering changes in all other body systems
Drugs do not produce new functions on tissues or organs of the body. Usually, a primary site of drug action is targeted by a drug that is administered systemically; nevertheless, all body tissues are affected in some way by every drug administered. The beginning and ending point of drug action is generally considered the pharmacodynamic effect. This describes the outcome effects after the drug reaches the site of action.
The intent of drug therapy is to produce a therapeutic effect that may be to control pain, cure a particular disease, alleviate symptoms of a disease, or diagnose a disease. The radiographer will be involved most of the time with drugs used to diagnose disease.
The particular area for which a drug is intended and that receives a maximum effect of a drug is called the drug receptor. The function of a cell is altered but not completely changed by a drug. A drug receptor is a macromolecular component of body tissue.
Drug receptors have an affinity for a particular drug. This means that there is an attraction between the drug and the receptor. Affinity is the factor that determines the concentration of drug necessary to accomplish its intended effect. If there is a strong affinity at the receptor site, the concentration of drug necessary to accomplish its effect is low. This is referred to as the efficacy of the drug.
There are both drug agonists and antagonists. These actually provide opposite reactions. Agonists are drugs that are able to bind with receptors to produce a therapeutic response. Partial binding at the receptor site is also possible to produce a partial therapeutic response. These are called partial agonists. A drug antagonist joins with a receptor and prevents the agonist from performing its intended effect.
The molecular structure of each drug determines the affinity for a receptor. A very small change in a drug molecule can leave the drug’s affinity to a receptor unchanged but drastically change the pharmacologic action of a drug.
Many drugs exert more than one effect on the body. For instance, a drug given at the recommended dosage will have the intended effect; however, if given in a larger dose, it may have an undesirable effect. The relation between the dosages at which the intended effect of a drug is obtained and the amount that produces an unwanted effect is called the therapeutic index. The greater the therapeutic index, the safer the drug is, because more can be tolerated without adverse effect.
ADVERSE DRUG REACTIONS
Any person who participates in drug administration must be aware of the potential harm that may result from drugs. They may produce many unintended effects. When a drug produces an effect that is mild, common, unintended, and nontoxic, it is called a side effect. When a drug produces an effect that is more severe or life threatening, it will be referred to as an adverse reaction. Some adverse reactions occur almost immediately after the drug is administered, and some take weeks or months of administration before an untoward reaction is produced.
A toxic reaction is an unwanted effect that is an extension of the therapeutic effect, such as an extension of the therapeutic effect. A drug given in the prescribed amount is therapeutic but may become toxic when given in an increased amount; this is considered as overdose. A toxic reaction does not include an allergic reaction or anaphylactic shock, which is classified as an adverse reaction.
Immediate adverse reactions to drugs can include gastric distress and allergic or hypertensive reactions that may range from mild urticaria to severe anaphylactic shock. Some drugs are disease-producing themselves and may produce blood dyscrasias, hepatic disease, gastric ulcerations, or thrombosis.
Because drugs are tested extensively before they are permitted to be marketed, most adverse reactions have been documented, and it is known that these reactions may occur. The therapeutic or diagnostic purpose of a drug is weighed against the risk factors prior to administration. If the need outweighs the risk, it is prescribed by the physician with caution.
The patient must be educated regarding the potential adverse effects of a drug before it is administered. He must be instructed to notify his physician if adverse reactions occur. Particular care must be given to pregnant women and nursing mothers. All health care workers who administer drugs have the responsibility to understand and educate their patients about any drug that they administer.
An unexpected or exacerbated effect from a drug is called a drug idiosyncrasy. For example, a drug given to produce sleep instead produces a hyperactive reaction. The cause of this reaction is not clear, but may be due to a genetic deficiency that creates an inability to tolerate certain chemicals.
Drug tolerance occurs when a drug received continually for a length of time creates a change in the response to that drug. Usually, the drug is needed in increasingly larger doses to create the desired effect. Drug tolerance is a sign of drug dependence, and the drugs that are related to this problem are narcotics and tranquilizers. Tachyphylaxis is a rapid development of tolerance to a drug.