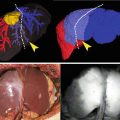
Fig. 30.1
(a) It is difficult to detect tiny lesion by conventional white light cystoscopy. (b) PDD cystoscopy prevents from overlooking of tiny lesion
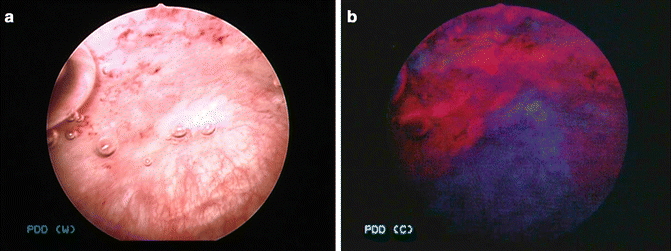
Fig. 30.2
(a) It is difficult to detect flat lesion by conventional white light cystoscopy. (b) PDD cystoscopy can adequately detect flat lesion such as dysplasia and carcinoma in situ
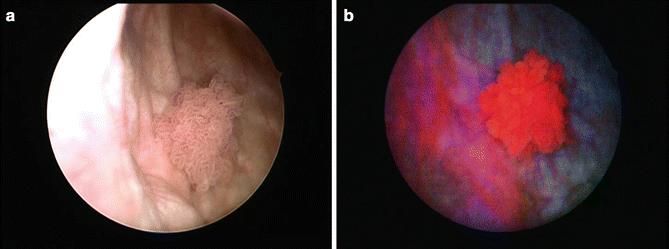
Fig. 30.3
(a) It is difficult to detect correct surgical margin by conventional white light cystoscopy. (b) PDD cystoscopy can confirm concomitant flat lesion with raised tumor
Table 30.1
Meta-analysis and systematic review of ALA-PDD
Author/year of publication (reference number) | Evidence synthesis and results | |||
|---|---|---|---|---|
Diagnostic trials | Randomized trials | |||
Number of assessed studies | Number of assessed studies | |||
Kausch I, et al. 2010 [13] | Higher additional detection rate (all tumors: 20 %, CIS: 39 %) | 12 studies | Less residual tumor (odds ratio: 0.28) and higher recurrent free survival (12–24 months) | 5 studies |
Mowatt G, et al. 2011 [14] | Higher sensitivity 92 % (versus WLE: 71 %) and lower specificity 57 % (versus WLE: 72 %) | 27 studies | Less residual tumor (relative risk: 0.37) and longer recurrent-free survival (relative risk: 1.37) | 4 studies |
Burger M, et al. 2013 [15] | Higher additional detection rate (Ta: 14.7 %, CIS: 40.8 %) | 10 studies | Higher recurrent-free survival (−12 months) | 3 studies |
Rink M, et al. 2013 [16] | Higher additional detection rate (Ta, T1: 7–29 %, CIS: 25–30 %) | 32 studies | Lower residual tumor rate (20 %) and improve recurrent-free survival but not progression-free survival | 13 studies |
Currently, ALA and ALA derivatives, such as an oral agent for brain tumors, Gliolan®, and an agent for intravesical administration, Hexvix®/Cysview®, have been approved as pharmaceuticals worldwide. Hexvix®/Cysview® was developed to increase the intratumor PpIX accumulation rate by converting water-soluble ALA to a fat-soluble ester compound as an intravesical agent, and many favorable outcomes have been reported. However, its application cannot be expanded to systemic administration because it is a hexyl ester hydrochloride exhibiting strong cytotoxicity.
Transurethral Resection of a Bladder Tumor (TURBT) Navigated by ALA-PDD Fluorescence
ALA-PDD is not only for diagnosis, but also plays an assistive role for surgical resection by identifying the tumor range. As described above, the standard treatment for NMIBC is TURBT. In TURBT, additional resection under navigation of ALA-PDD fluorescence can avoid insufficient resection of tumors (see Video 30.2), reducing the rate of postoperative intravesical recurrence.
Regarding the recurrence-free survival rate, Denzinger et al. [18–20] compared the intravesical recurrence rate after TURBT over a long-term follow-up for a maximum of 8 years involving 191 bladder cancer patients. The recurrence-free survival rates after PDD-TURBT were significantly and consistently higher than that after conventional TURBT. They also performed multivariate analysis employing the Cox proportional-hazards model, in which the hazard ratio of PDD-TURBT was 0.29 (95 % confidence interval: 0.15–0.56, p = 0.0002), showing that PDD-TURBT was an independent prognosis-improving factor related to intravesical recurrence. In our clinical experience [13], the recurrence-free survival rate over a median duration of follow-up of 22 months (0.2–68.7 months) in 99 non-muscle invasive bladder cancer patients was 86.9 % after 12 months, 74.7 % after 24 months, 69.7 % after 36 months, 67.7 % after 48 months, and 66.7 % after 60 months, showing that the rate was significantly increased compared to that after conventional white light TURBT. We also performed multivariate analysis employing the Cox proportional-hazards model. The hazard ratio of PDD-TURBT was 0.578 (95 % confidence interval: 0.371–0.888, p = 0.012), showing that PDD-TURBT was an independent prognosis-improving factor related to intravesical recurrence. Moreover, meta-analysis and systematic review showed less residual tumor and improve recurrent-free survival but not progression-free survival in randomized trials of ALA-PDD (see Table 30.1) [14–17].
Adverse Events
In ALA-PDD, adverse events, such as phototoxic reactions, mainly photosensitive dermatitis, and liver disorder, induced by previous photosensitive substances, such as hematoporphyrin mixtures and porphyrin derivatives, is of the most serious concern. However, as described above, ALA is a natural amino acid contained in animals and plants, and it is less toxic, highly cancer-specific, and rapidly metabolized and excreted by normal cells. When ALA was systemically administered orally, the administered ALA was mostly eliminated from plasma after 6 h and excreted from the body after 24 h [21]; whereas, when ALA was administered into the bladder, the maximum plasma level of the predisposing cause of ALA-induced adverse events, protoporphyrin IX (PpIX), about 30 min after intravesical administration was about 1/100 of that after oral administration, and the half-life was also short (about 54 min) [22]. It is pharmacologically impossible for these conditions to cause phototoxic reactions, mainly photosensitive diseases, and liver disorder.
Actually, no photosensitive dermatitis developed, requiring no protection from light, in not only these pharmacological verifications but also an intervention study reported by Filbeck et al. [23], in which patients treated with intravesically applied ALA underwent experimental ultraviolet light irradiation, and a study involving the largest number of bladder cancer patients diagnosed by PDD using ALA (1,713 applications in 875 patients). In our clinical experience involving the 210 bladder cancer patients [13], although no special precaution, such as liver support and light shielding, was implemented throughout PDD, bladder irritation symptoms, such as pollakiuria and urgency, were observed after intravesical ALA administration in 17.3 %, photosensitive diseases after oral ALA administration in 4.4 %, elevation of blood liver enzyme levels [Aspartate Aminotransferase (AST), Alanine Amino-transferase (ALT)] in 3.0 %, and nausea/vomiting in 3.0 %, but all of these events were mild and transient, and no serious adverse event occurred.
Conclusion
ALA-PDD is a clearly well-established navigation system of diagnosis for urinary bladder cancer, particularly CIS. Two major academic societies, European Association of Urology [24, 25] and American Urological Association [26], already showed the specific indications and also the general recommendations of ALA-PDD for urinary bladder cancer in their guidelines. In also Japan, it is hoped the establishment of fluorescence-guided navigation system based on ALA-PDD in urinary bladder cancer.
Moreover, photodynamic technology employing ALA is based on the common biological characteristic of cancers, which may be clinically applicable for not only bladder cancer [7, 11–20, 24–26], but also various other forms of cancer, such as prostate cancer [8, 9], renal cancer [10], brain tumors [27], pharyngo-laryngeal cancer [28], gastric cancer [29–31], colon cancer [32], rectal cancer [32], lung cancer [33], pleural malignancies [34], uterine cervical cancer [35], skin cancer [36], and spinal meningioma [37].