Fig. 20.1
Photodynamic diagnosis system. An endoscopic photodynamic diagnosis system comprising a straightforward telescope, light source, and a video camera system
Photosensitive Substance
PDD imaging systems were recently improved to enable detection of premalignant and early-stage malignant lesions in the lung and esophagus based on systemic administration of photosensitizing drugs, which are mostly porphyrin compounds such as Photofrin [7, 8]. However, the use of Photofrin has considerable disadvantages, such as strong phototoxic skin reactions that can persist for weeks.
5-Aminolevulinic acid (ALA) is a naturally occurring amino acid derivative that acts as an endogenous substrate and precursor to PpIX in the heme biosynthetic pathway. Although the underlying mechanism is not well known, exogenously administered ALA increases cellular levels of PpIX in a number of tissues, particularly at epithelial surfaces, and higher accumulation of PpIX occurs in tumor cells than in normal cells [5, 9, 10]. PDD using ALA (ALA-PDD) is clinically recognized as an effective procedure for detecting various cancers including brain tumor, bladder cancer, and prostatic cancer [3, 11, 12]. ALA offers several advantages as a photosensitizer for PDD, including the low risk of side effects; indeed, increases in serum aminotransaminase levels are only temporary and skin phototoxicity is limited to 1 or 2 days with ALA [2, 3, 13].
In our experience, no special precautions have been necessary during ALA-PDD, such as liver support or light shielding, and no adverse events were encountered. However, although ALA-PDD is an officially approved diagnostic procedure in Europe for detecting bladder cancer, many problems remain concerning the accuracy of diagnosis due to false-positive fluorescence and photobleaching during the procedure. Thus, we investigated using ALA as a photosensitizer for PDD in gastric cancer. ALA hydrochloride (Cosmo Bio Co., Ltd., Tokyo, Japan) was dissolved in 50 mL of 5 % glucose solution and 1.0 g of this solution was given orally through a stomach tube 3–4 h before the intraoperative PDD observation. Patients were shielded from direct sunlight for 24 h to avoid phototoxicity.
PDD for Gastric Cancer
Gastric cancer is the fourth most common cancer and second most common cause of cancer-related death worldwide [14]. It is the most prevalent form of cancer in Japan and East Asia, and the treatment of gastric cancer often requires gastrectomy with sufficient lymphadenectomy [15]. Surgical treatment achieves good rates of disease control, particularly in the early stages of gastric cancer [16], and the 5-year overall survival rate in Japanese gastrectomy patients with Stage I gastric cancer exceeds 90 % [17, 18]. Generally, diagnosis of gastric cancer relies on endoscopy and subsequent histological examination of tumor biopsies; however, several difficulties in diagnosis remain with regard to determining the extent of cancer infiltration in some carcinomas including the absence of typical morphological structures and indistinct margins [19]. Although esophagogastroduodenoscopy (EGD) is highly reliable in defining the area of cancer infiltration in early gastric cancer, it remains difficult to diagnose gastric cancer accurately in some cases such as gastric-type differentiated adenocarcinoma if biopsy specimens do not reveal the presence of adenocarcinoma [20, 21]. Only a few studies using ALA-PDD have been reported for cases of gastric cancer, and the clinical diagnostic accuracy and sensitivity of this approach as an alternative for diagnosing gastric tumors remain unclear [6, 22, 23].
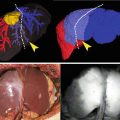
Recently, we reported the feasibility and efficacy of intraoperative ALA-PDD for the identification of gastric cancer [4]. Intraoperative PDD using 1.0 g of ALA solution given orally through a stomach tube was carried out for 26 lesions of 21 patients with gastric cancer. Figure 20.2 shows a 71-year-old female who underwent proximal gastrectomy. Our ALA-PDD system revealed a slightly elevated lesion in this patient with a distinct margin demarcated by an intense fluorescent signal (Fig. 20.3 and Video 20.1). This lesion was pathologically diagnosed as well-differentiated adenocarcinoma confined to the mucosal layer.
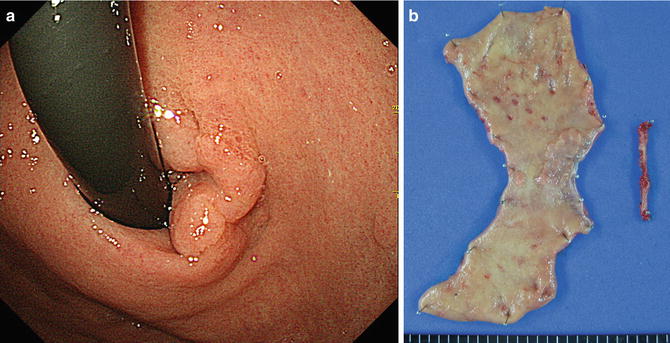
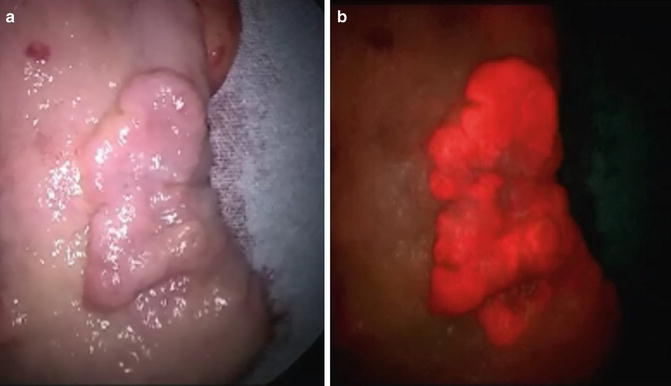

Fig. 20.2
Early gastric cancer based on photodynamic diagnosis. Esophagogastroduodenoscopy showed an early gastric cancer located in the cardia near the esophagogastric junction (a). The patient underwent proximal gastrectomy (b)

Fig. 20.3
Photodynamic diagnosis findings of gastric cancer compared to those using white light. Under white light, the tumor appeared as a slightly elevated lesion (a). Under ALA-induced fluorescence, the tumor emitted a red fluorescence signal that was consistent with the area of adenocarcinoma defined by pathological examination (b)
Gastric carcinoma has been classified into two major histological types, intestinal and diffuse, according to the Lauren classification system [24]. In our studies, the incidence of intestinal-type tumor was significantly higher in PDD-positive than in PDD-negative cases (93.3 % vs. 27.3 %; P < 0.001). Specifically, we found that significantly more ALA-PDD positive lesions were intestinal-type than diffuse-type gastric cancer. Interestingly, this finding could reflect differences in the porphyrin biosynthesis pathway at play in different histological types of gastric cancer [25–27].
Red fluorescence was detected in 15 lesions of 11 patients, and the sensitivity, specificity, positive predictive value, negative predictive value, and accuracy of ALA-PDD in detecting gastric cancer was measured at 57.7, 100, 100, 38.9, and 66.7 %, respectively (Table 20.1). The sensitivity and accuracy of ALA-PDD for detecting gastric tumors was therefore low in our clinical application of this modality to gastric cancer patients, and although the sensitivity of ALA-PDD for intestinal-type lesions was high, it was low for diffuse-type gastric cancer. Among only intestinal-type gastric tumors, the sensitivity, specificity, positive predictive value, negative predictive value, and accuracy of ALA-PDD in detecting gastric cancer was measured at 82.4, 100, 100, 87.5, and 70.0 %, respectively (Table 20.1).
Table 20.1
Diagnostic ability for ALA-PDD in gastric cancer
Sensitivity (%) | Specificity (%) | Accuracy (%) | PPV (%) | NPV (%) | |
|---|---|---|---|---|---|
Intestinal type | 82.4 | 100 | 87.5 | 100 | 70 |
Total | 57.7 | 100 | 66.7 | 100 | 38.9 |
Mechanism of PPIX Accumulation in Cancer Cells
The oligopeptide transporters (PEPT) such as peptide transporter 1 and 2 (PEPT 1 and 2) are reportedly involved in the cellular uptake of ALA [28–30]. Two molecules of ALA are converted to porphobilinogen, which is metabolized to porphyrinogen intermediates by porphobilinogen deaminase (PBG-D). The last step of metabolism for ALA is the incorporation of iron into PpIX, catalyzed by ferrochelatase (FC) [5]. Previous studies reported that some cancer cells have an increased PBG-D activity [31, 32] and a decreased FC activity [31, 33]. Therefore, ALA-induced PpIX accumulation is possibly due to the limited capacity of FC or the higher activity of PBG-D in tumors [34].
Recent studies also suggested that the expression of particular proteins could affect ALA-mediated PpIX accumulation in tumors, including PEPT1 and 2, FC, and ATP-binding cassette transporter G2 (ABCG2) [25, 26, 35]. Indeed, PEPT1 and ABCG2 showed pivotal roles in regulating intracellular PpIX levels and cellular photosensitivity in gastric cancer cells treated with ALA [25]. Experiments in lung adenocarcinoma, renal cancer, and colorectal cancer cell lines also indicated that mitochondrial ABCG2 might regulate ALA-mediated PpIX levels through exporting it from mitochondria to the cytosol [26]. In a gastric cancer cell line, high expression of PEPT1 and low expression of ABCG2 correlated with ALA-induced PpIX production and cellular photosensitivity [25]. Distribution of biomarkers including PEPT and ABCG depending on histological type might therefore underlie the observed differences in ALA-PDD positivity among gastric cancers.
Future Direction of PDD for Gastric Cancer
It is expected that PDD will be useful for the following future directions (Table 20.2). First, it could be used a tool for evaluating surgical resection margin, and thereby assisting the pathological diagnosis during surgery. Diagnostic ability without any adverse events is consistent with a recent study of ALA-PDD showing outstanding sensitivity for the detection of superficial bladder cancer, particularly for flat lesions such as dysplasia and carcinoma in situ without additional complication [3]. Superficial spreading-type cancer of the stomach also has some specific clinicopathological characteristics compared to the common type of gastric cancer [17]. For instance, in a case of duodenal invasion of early gastric cancer, the invasion was more extensive in superficial spreading lesions than in small cancer lesions [36, 37]. In such cases, ALA-PDD might be useful in determining the extent of gastric adenocarcinoma in cases with indistinct margins, and provide additional information when judging the sufficiency of proximal or distal margins during surgery.
Table 20.2
Feature direction of photodynamic diagnosis for gastric cancer
• Evaluation of surgical resection margin |
– Assist pathological diagnosis during surgery |
• Evaluation of lesion extension |
– Supplemental modality to image-enhanced endoscopy and magnifying endoscopy |
• Diagnosis of peritoneal metastasis during preoperative staging laparoscopy |
– Provide useful information for selecting a therapeutic modality |
Moreover, it is expected that ALA-PDD could provide not only early detection of gastric cancer, but also a multimodal diagnostic tool for use during less invasive therapies such as endoscopic mucosal dissection (ESD). In such situations, ALA-PDD might prove valuable for evaluating the lesion extent as a supplemental modality to image-enhanced endoscopy and magnifying endoscopy. In Japan, ESD is commonly performed to treat early gastric cancer, allowing en bloc resection [38, 39]; however, it is important to detect any extension of the cancer [37, 40] and in this regard, ALA-PDD could provide additional information to that obtained with conventional endoscopic observation.
Another future direction for ALA-PDD is in the diagnosis of peritoneal metastasis during preoperative staging laparoscopy. Peritoneal metastasis caused by the seeding of exfoliated cancer cells from the primary gastric cancer is the most common type of spread in advanced gastric cancer with serosa-invading tumors [15, 41]. Although staging laparoscopy is used frequently in the management of patients with advanced gastric cancer to prevent unnecessary laparotomy [42, 43], it has limitations in visualizing dissemination of the cancer nest [44]. To this end, some investigators reported the effectiveness of ALA-PDD for early endoscopic detection of malignant esophageal lesions, and for the staging laparoscopy of advanced gastric cancer due to improvements in the detection sensitivity for peritoneal metastases [6, 13, 22]. Thus, ALS-PDD could also provide information that aids in selecting a therapeutic modality.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree