, Foster D. Lasley2, Indra J. Das2, Marc S. Mendonca2 and Joseph R. Dynlacht2
(1)
Department of Radiation Oncology, CHRISTUS St. Patrick Regional Cancer Center, Lake Charles, LA, USA
(2)
Department of Radiation Oncology, Indiana University School of Medicine, Indianapolis, IN, USA
Definitions
AAPM TG–43: Task Group Report 00 of the American Association for Physics in Medicine.
Activity (A): Amount of radioactive material.
Units: 1 Curie (Ci) = 3.7 × 1010 Becquerel (Bq)
Radius (r): aka distance, depth.
Dose Rate (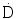 ): not to be confused with total dose (D)
): not to be confused with total dose (D)
Initial Dose Rate (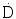 0 )
0 )
Exposure Rate Constant (Γ), aka Gamma Constant: Exposure rate per millicurie of isotope at 1 cm distance.
Exposure Rate (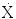 ) = ΓA
) = ΓA
Milligrams Radium Equivalent (mgRaEq):
1 mgRaEq = 8.25 R–cm 2 –h −1 –mg −1 exposure rate
Air kerma strength (S K ): Kerma (kinetic energy released in matter) measured in air @ 1 m.
1 U = 1 cGy–cm −2 –h −1 = 1 μGy–m −2 –h −1
Proportional to Activity.
Dose rate constant (Λ): Dose rate to water for 1U air kerma strength at 1 cm (cGy-cm−2-h−1/U).
Low Dose Rate (LDR): 0.4–2 Gy/h
Medium Dose Rate (MDR): 2–12 Gy/h
High Dose Rate (HDR): >12 Gy/h
Pulse Dose Rate (PDR): HDR fractionated over time to approximate LDR dose rates.
Details of the units and activities along with dosimetry can be found in AAPM TG–43.
The Historical Role of Radium
226 Ra brachytherapy was used for many decades prior to 60Co, 137Cs, 192Ir, or megavoltage X-rays.
Radium sources consist of radium chloride powder placed within a double-sealed platinum tube.
226 Ra comes to a secular equilibrium with 222 Rn and its decay products by emitting alpha rays.
This results in accumulation of multiple radioactive daughter nuclides emitting alphas, betas and gammas.
The encapsulation is designed to absorb everything except for the gammas.
Average photon energy 0.83 MeV (range 0.18–2.29 MeV).
226 Ra is no longer used because of the risk of radon gas leakage and other safety concerns.
Many LDR brachytherapy systems are based on “milligrams radium equivalent” (mgRaEq).
For a source of activity A and gamma constant Γ:
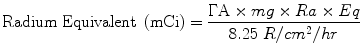
(11.1)
Commonly Used Therapeutic Radionuclides
Common sealed source nuclides include 226 Ra, 192 Ir, 137 Cs, 125 I, 103 Pd, and 60 Co.
Common unsealed source include 131 I, 90 Y, and 32 P.
Nuclides are chosen for their desired characteristics such as type of radiation, half-life, energy, etc.
125 I and 103 Pd emit very low energy characteristic X-rays (22–28 keV); this gives them a very steep dose fall-off.
The other sealed source nuclides are high energy gamma emitters.
Most unsealed source nuclides emit short range beta radiation with a short half-life, thus limiting the risk of systemic and environmental contamination.
Refer to Appendix B for a list of nuclides used in imaging and therapy.
Production of Radionuclides
Naturally Occurring: Byproducts of uranium decay, these nuclides can be mined from the Earth.
226 Ra, 223 Ra, 222 Rn among others.
Fission Byproduct: Obtained from nuclear reactors.
137 Cs, 131 I, 90 Sr among others.
Neutron Bombardment: Creates beta-minus emitters. Cyclotrons can produce high intensity proton and neutron flux. Nuclear reactors can produce very high intensity neutron flux.
198 Au, 192 Ir, 153 Sm, 125 I, 103 Pd, 89 Sr, 60 Co, 32 P among others.
Proton Bombardment: Creates beta-plus emitters, often used for PET imaging. Protons are accelerated by a cyclotron.
123 I, 18 F, 15 O, 11 C, 3 H among others.
Daughter Elution: A longer-lived mother nuclide (“cow”) decays into a shorter-lived daughter nuclide (“milk”) that can be repeatedly eluted for clinical use. This is an example of transient equilibrium.
90 Y, 99m Tc among others.
Sealed Source Properties
Classically, source strength is measured as activity (Ci or Bq) or milligrams radium equivalent (mgRaEq).
Two sources with the same Activity (Ci) may emit very different amounts, energies and types of radiation due to encapsulation and filtration. Hence, their dose rate may be different.
Source strength is specified as air kerma rate at a distance of 1 m as mentioned above. (1 U = 1 μGy/h/m 2 ).
Unsealed Source Properties
Unsealed sources do not have to worry about encapsulation so they simply are specified as nuclide, activity, and chemical formulation. (ie elemental vs. colloidal vs. antibody-bound).
An unsealed source will have separate physical and biological half-lives.
Effective half–life equation:
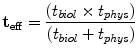
(11.2)
See Chapt. 2 for more half-life equations.
Implant Instrumentation and Technique (Ircu-38 and 58)
An intracavitary implant is placed within an applicator such that the sources do not directly contact tissue.
Tandem and ovoids (ie, Fletcher-Suit)
Ring and tandem
Vaginal cylinder
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree