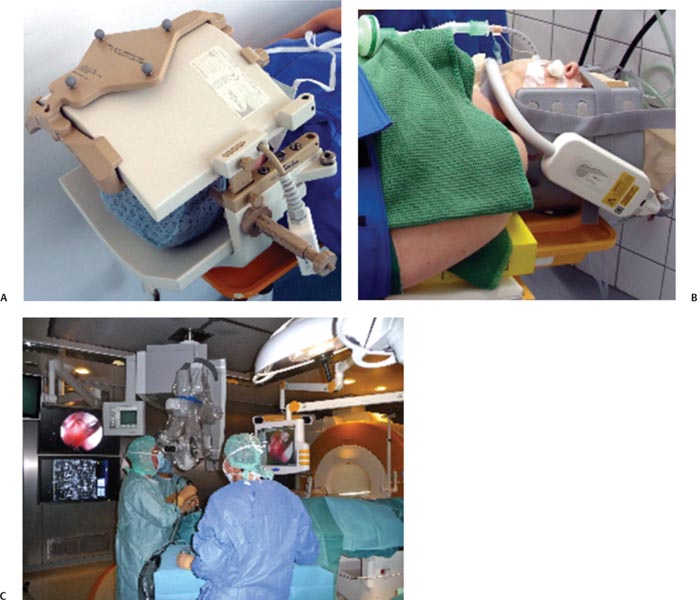
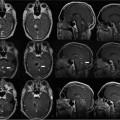
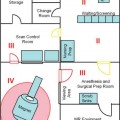
13 Despite ongoing advances in pharmacologic treatment and radiotherapeutic management, especially of hormonally active pituitary adenomas, surgery remains the therapy of choice for the large majority of these tumors and it is the first treatment of choice in hormonally inactive tumors. Among the advanced technologies in transsphenoidal surgery, neuronavigation,1–6 endoscopy,7,8 and especially intraoperative magnetic resonance imaging (iMRI)1,9–16 are remarkable adjuvant techniques that have been introduced into the operating room (OR) in recent years. Intraoperative imaging is used as an immediate intraoperative quality control, evaluating the extent of tumor removal during the surgical procedure and allowing us to extend resections in those cases where tumor remnants are documented.9–12,14–16 Using low-field and especially high-field 1.5 tesla (T) iMRI scanners during the surgical treatment, it’s possible to find that up to one-third of pituitary macroadenomas, suprasellar tumor remnants as small as 3 to 4 mm, as well as larger adenoma remnants were hidden to the surgeon’s eye in folds of the descending sellar diaphragm. The first intraoperative imaging modality used during transsphenoidal surgery was x-ray fluoroscopy,17 limited by the fact that only bony structures of the cranium were visible. In an early attempt to estimate indirectly the extent of tumor removal, intraoperative gas cisternography was used. First attempts for a direct evaluation of the effects of transsphenoidal surgery started in the 1980s with the application of ultrasound18,19 and computed tomography (CT).20 Unfortunately, these early attempts were not encouraging because of the restricted quality of the intraoperative images. In the mid-1990s, the development of an open MRI system made iMRI possible for the first time. This application was pioneered by Black et al,21 who operated in a continuously running magnetic field using the so-called double doughnut system. In the 0.2 tesla open-MRI installations in Erlangen and Heidelberg, the patient was transported into the magnet, while surgery was performed outside the strong magnetic field. The evolution from low-field MRI scanners to high-field MRI scanners definitely revolutionized intraoperative imaging in transsphenoidal surgery with regard to resection control and high-quality intraoperative images. High-field MRI has a clear advantage in image quality compared with the intraoperative low- and midfield MRI systems (0.12–0.5 T).22 Not only patients with large tumors but also smaller adenomas, especially developing against and into the cavernous sinus can profit from iMRI control. Morphologically radical tumor resection is a prerequisite for endocrine remission in normally smaller hormonally active tumors, in acromegaly, and Cushing disease. iMRI increases the rate of complete tumor removal and endocrine normalization and improves endocrine outcome to “nearly normalization.”23 In the case of a biochemical cure of growth hormone secreting tumors in acromegaly, follow-up MRI scans may not even be required if endocrine parameters are controlled because no tumor remnant will be visible.24 In nonfunctioning tumors, there are no hormonal markers available that would allow an early prognosis of outcome. A morphologic assessment of the radicality of the tumor resection is still required as the most sensitive diagnostic tool. Because of immediate postoperative artifacts, this information can only be gained some 2 to 3 months after the operation.25 Today different industry companies offer low-field (0.15 T -Medtronic Navigation, Inc., Louisville, CO; 0.2 T – Siemens AG, Erlangen, Germany), mid-field (0.3 T – Hitachi Medical Corp., Tokyo, Japan; 0.5 T – General Electric Medical Systems, Waukesha, WI), high-field (1.5 T – General Electric, Philips Healthcare, Andover, MA], Siemens), and ultra-high-field (3.0 T – General Electric, Philips, Siemens). The senior author (RF) started with 0.2 T system (Magne-tom Open, Siemens) in 1996, followed by 1.5 T system (Sonata, Siemens) in 2002 in Erlangen. We now present our actual system, the INI Brain Suite, an operating theater with a 1.5 T Magnetom Espree scanner (Siemens), which was installed in 2006/2007 at the International Neuroscience Institute INI (Hannover, Germany). This is a high-field MRI scanner with a superconductive 1.5 T magnet with a length of 160 cm and an inner bore diameter of 70 cm equipped with a gradient system with a field strength of up to 40 millitesla per meter (mT/m; effective 69 mT/m) and a slew rate of up to 200 T/m/s effective. This was the first “open” 1.5 T MRI, used for therapeutic purposes. A rotatable surgical table (Trumpf, Saalfeld, Germany) is adapted to the scanner to allow for a special surgical MRI tabletop. This surgical table can be locked into various positions. The principal surgical position is at 160 degrees with the patient’s head at the 5 gauss (G) line (distance of 4 meters to the center of the scanner). As soon as the rotating mechanism has been locked, the height of the table, the angle of tilt, and the lateral tilt can be modified. The table movements are controlled remotely. Only the rotation about the table axis to turn the table into the axis of the scanner is performed manually, for safety reasons. MRI-compatible ventilation (Aestiva 5/MRI, General Electric, Hannover, Germany) and MRI-compatible monitoring are available for control of anesthesia and for wireless 2.4 GHz data transfer from the radiofrequency- (RF-) shielded cabin. The perfusion and infusion pumps are shielded for MRI compatibility. After starting with the NC4 Zeiss Multivision microscope, which was installed at the left side of the head, outside of the 5 G line, we are working now with the ceiling mounted Pentero C Multivision microscope (Zeiss, Oberkochen, Germany). The holding device is placed in the middle line within the 5 G line, the microscope itself can have a flexible position in connection with the preferred position of the operating field (to the best of our knowledge, we tested this MRI-compatible ceiling-mounted Zeiss Pentero system for the first time). If navigation is indicated in transsphenoidal surgery (encased arteries, loss of anatomic landmarks in second operation), we use the BrainLab integrated VectorVision Sky Navigation System (BrianLab, Heimstetten, Germany) with roof-fixed infrared camera and touch screen display. The patient is fixed in a special ceramic head holding system (Fig. 13.1A) and the registration is done automatically through an integrated special coils system. A fiberoptic connection ensures MRI-compatible integration into the RF room. The camera used to monitor the positions of the microscope and other instruments is ceiling-mounted, as is the touch screen, which is used to operate the navigation system. Two 50-inch flat-screen monitors (Braco) mounted on the left wall of the Brain Suite are available for viewing the images from the microscope and the MRI console, as well as various software applications. The microscope videos are documented using Medimage software (Vepro, Pfungstadt, Germany) and in parallel in the MRI control room. The electric current to the microscope and to other devices that may interfere with the MRI signal is automatically switched off during MR imaging. The 0.5 mT and 20 mT lines are marked on the floor. The 20 mT line also is marked with a raised stainless steel strip as a physical threshold. The instruments table and the various rotating stools (Trumpf) are fully MRI compatible. The procedures for emergency magnet quenching and for monitoring of oxygen levels in the operating room are the same as those in standard clinical MRI installations. In general, the great majority of tumors operated via transsphenoidal approach do not require head fixation. Imaging is performed using a standard U-shaped large flexible coil that is adapted and draped to the head (Fig. 13.1B). The surgeon, who prefers Cushing’s positioning for transsphenoidal surgery, is standing behind the patient’s head in Fig. 13.1C. A schematic outline of the operating room was published previously.11,26 The whole transsphenoidal procedure is identical to that performed in regular operating rooms. Besides the sublabial and unilateral paraseptal approach to the sphenoidal sinus, we prefer in general the direct pernasal transsphenoidal approach with endoscope assistance. Porcelain-coated drills are used – especially to avoid metal artifacts during imaging -to open the sphenoidal sinus and to remove the sellar floor. Transsphenoidal surgery is routinely accompanied by en-donasal endoscope using 0 degree and 30 degree, 4-mm rigid endoscopes by visualizing the surgical site on a ceiling-mounted monitor or in the eyepiece of the microscope. Selective adenomectomy was performed in all patients. In general, regular operating micro instruments are used during the transsphenoidal surgery at the 5 G line. In contrast to earlier years when we used MRI-compatible nasal speculum for intraoperative imaging, the direct endoscopic-assisted endonasal approach allows us examinations without the speculum, just installing simple cotton as a place holder in the nasal cavity. If necessary, especially in cases with defects of diaphragm sellar and cerebrospinal fluid (CFS) leaks, the sellar floor was covered at the end of surgery with muscle fascia and/or subcutaneous fat tissue, which was obtained during the same surgical session from the right thigh positioned at the border of the 5 G perimeter. Therefore, in the case of intraoperative CSF leakage, a transitory lumbar drainage was administered. In direct pernasal surgery, no nasal packing is necessary. The timing of intraoperative imaging is decided by the neurosurgeon; intraoperative imaging is performed either when he has the impression of complete tumor removal or, in the case of incomplete tumor removal, when he thinks no further removal at this stage of surgery is possible using the transsphenoidal approach. Just before intraoperative imaging, the opened sellar floor is covered with a flat piece of bone wax for better delineation of the sellar outlines in the intra-operative images, minimizing artifacts caused by blood from the sphenoid sinus or the nasal cavity. Then, the surgical site is covered with a sterile drape, and the MRI table is rotated 160 degrees into the scanner. The time between the decision for iMRI and the actual start of imaging is ~2 minutes. Fig. 13.1 (A) Rigid navigated magnetic resonance (MR) coil is used to fix the head during all surgical procedures. (B) Flexible MR coil is placed around the head, so the head can be moved during surgery without restrictions; a coil-preamplifier is attached to the flexible coil with tape. (C) Intraoperative scenario of our MR imaging (MRI-) operating room for microneurosurgical procedures. The head is placed at the 5 gauss red line; the surgeon is standing behind the head (Cushing’s position). The microscope and its associated monitor are fixed on the roof of the operating theater, on the left side of the surgeon; the instrument tables with endoscope and as well the nurses are stationed on the right side of the surgeon. The anesthesiologist’s workstation is located in the right corner of the operating theater, opposite the MRI table end. The circuit for the endotracheal tube is connected to an MRI-compatible anesthesia machine. Many standard surgical instruments can be used between the 5- and 20-gauss magnetic field lines. Two 50’ plasma monitors are collocated on the left wall of the operating theater. A movable MRI-compatible nawgation monitor and infrared navigation arm is located close to the left middle part of right wall of the operating theater. An extra 21’ liquid crystal display (LCD) monitor is fixed on the roof on the left side of the operating theater.
Pituitary Tumor Resection-iMRI in Transsphenoidal Surgery
Operating Room Set-up
Transsphenoidal Surgery and iMRI
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree