Chapter 7 Planning and Guiding Mitral Valve Repair
Introduction
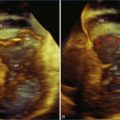
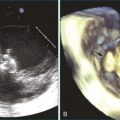
Mitral valve prolapse is the most common structural cause of chronic mitral regurgitation leading to surgery in developed countries. The prevalence of mitral valve prolapse has been reported to be 2.5%.1 Mitral valve repair is the clear treatment choice for symptomatic severe mitral regurgitation and now asymptomatic mitral regurgitation in many cases. An important advance in assessing mitral valve disease has been the development and application of real-time three-dimensional echocardiography (RT3DE), particularly RT3D transesophageal echocardiography (RT3DTEE), which allows accurate localization of segmental anatomy and pathology of the mitral valve. This chapter provides an overview of the pathophysiology, anatomy, variants, and natural history of mitral valve prolapse and the clinical applications of RT3DE for mitral valve repair.
Background
Mitral valve prolapse is defined echocardiographically as superior displacement of the mitral leaflets into the left atrium by at least 2 mm beyond the mitral annular plane. Histologically, there is myxomatous degeneration of the leaflets, resulting in excessive and redundant leaflet tissue with elongated or ruptured chordae. The result is leaflet prolapse beyond the mitral annular plane during systole, causing regurgitation. This may lead to annular dilation and further progression of mitral regurgitation over time. There is a wide spectrum of mitral valve prolapse based on leaflet morphology. In the classic and more common form of mitral valve prolapse, the leaflets are diffusely thickened and redundant as a result of myxomatous degeneration. Such myxomatous changes may be localized to a single segment or involve the entire valve. The latter situation is known as Barlow disease, in which there is generalized thickening and billowing of the leaflets. In the less common, nonclassic form, the leaflets are normal in thickness. Fibroelastic deficiency is a term used when the disease is localized to isolated areas of the valve. Repair of Barlow disease can be challenging, with mitral valve repair techniques ranging from excision of excessive leaflet tissue to chordal manipulation and retention of leaflet tissue.2 RT3DTEE has provided quantitative and mechanistic analysis of Barlow’s disease versus fibroelastic deficiency, confirming the annular size and leaflet height in Barlow’s disease.3 Mitral valve prolapse appears to be both sporadic as well as familial. The familial cases appear to be inherited in an autosomal dominant fashion with variable expression. Mitral valve prolapse has been mapped to three genetic loci.4,5
The natural history of mitral valve prolapse is heterogeneous but largely determined by the severity of mitral regurgitation. Although most patients remain asymptomatic and may have a near-normal life expectancy, approximately 5% to 10% eventually develop severe mitral regurgitation.6,7 Without surgical treatment, mitral valve prolapse with severe mitral regurgitation results in left ventricular dysfunction, heart failure, atrial fibrillation, and pulmonary hypertension. Other serious complications include spontaneous rupture of the chordae, endocarditis, and stroke. Patients with mitral valve prolapse and severe mitral regurgitation have mortality rates of 6% to 7% per year if left unoperated, and approximately 10% per year progress to meeting clear surgical indications.8,9
Unlike mitral valve prolapse, functional mitral regurgitation is caused by geometric ventricular remodeling without any valve leaflet pathology. This condition typically is secondary to ventricular dilation from chronic ischemic mitral regurgitation or dilated cardiomyopathy. In chronic ischemic mitral regurgitation, the valve leaflets and chordae appear relatively normal, without chordal elongation or rupture. Closure of the mitral valve leaflets is restricted by tethering of the leaflets caused by papillary muscle displacement.10
Mitral valve repair frequently is performed for both degenerative disease and functional disease. However, repair rates have been disappointingly low. Only 44% of patients in the United States and 46% of patients in Europe who required surgery for mitral regurgitation received mitral valve repair, but the rates of repair have been increasing over time.11,12 Repair rates are lowest among patients with multiple comorbidities, those in New York Heart Association (NYHA) class III to IV, and those whose surgery is emergent.12 The listed rates of 44% and 46% in the United States and Europe, respectively, were published in 2003 and hence represent surgical practices in the late 1990s. In the decade starting in 2010, the standard for mitral valve repair is approaching 75% in some institutions.13 RT3DTEE has clearly played a role in this improvement, but there are many factors involved.
Use of Two-Dimensional and Three-Dimensional Echocardiography in Mitral Valve Repair
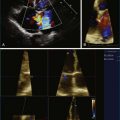
Noninvasive imaging, particularly echocardiography, plays a critical role in the initial and serial assessment of patients with chronic mitral regurgitation. A comprehensive echocardiographic examination is essential to a thorough understanding of the etiology and pathology of mitral regurgitation.14 A systematic approach has been previously described to interrogate the mitral valve with two-dimensional TEE (2DTEE).15 By using a standard approach to obtain four mid-esophageal views (four-chamber, bicommissural, two-chamber, and long-axis views) and the transgastric basal short-axis view, both mitral leaflets and their corresponding segments are carefully interrogated to determine the underlying pathophysiology of mitral regurgitation. The severity of mitral regurgitation can be determined by qualitative or quantitative Doppler assessment. The left ventricular end-systolic dimensions and ejection fraction can be accurately assessed by 2D and 3D techniques, although the assessment is more accurate by 3D volumes. With RT3DTEE, the mitral valve can be easily obtained in an anatomically correct orientation. The surgeon’s view also can be easily displayed by rotating the aortic valve to the 11 o’clock position. Compared with 2DTEE, 3DTEE is able to provide more accurate assessment of the annular dimensions and height, the leaflet length and surface area, and the coaptation area. The distances between the commissures and papillary muscles also can be measured. Such measurements are important for understanding the mechanism of mitral regurgitation and improving surgical planning of mitral valve repair. In a large cohort of patients undergoing mitral valve repair, 3DTEE has provided an objective means of predicting repair complexity in mitral regurgitation with a range of etiologies.16 The most predictive model for complex mitral valve repair includes multisegment pathology, prolapsing height, and posterior leaflet angle. These simple parameters are suggested to be objective indicators to guide surgical decisions about mitral valve repair. 3DTEE also has been shown to have the best agreement in identifying anterior leaflet prolapse compared with other echocardiographic techniques.17
Why Mitral Valve Repair?
Despite the major limitation of a finite incidence of recurrence of mitral regurgitation, mitral valve repair results in significant improvement in long-term survival compared with mitral valve replacement.18 The goals of mitral valve repair are to obtain an adequate surface of coaptation of both leaflets in systole and to preserve or repair leaflet mobility and the subvalvular annulus. In addition, an annuloplasty ring or band should be placed to reinforce the repair to correct and prevent progressive annular dilation.19,20 Carpentier’s technique remains the most common approach and is associated with excellent long-term results. It generally involves resection of abnormal or pathologic tissue, followed by precise reconstruction to restore normal valve anatomy.21 In patients with isolated prolapse of the posterior middle scallop (P2), the most common cause of degenerative mitral regurgitation, repair usually is treated with limited quadrangular or triangular leaflet resection and the removal of the elongated or ruptured chordae and supporting apparatus. The remaining scallops of the posterior leaflet are then reapproximated. To correct excessive posterior leaflet tissue, a sliding annuloplasty is performed to reduce the height of the posterior leaflet, followed by reapproximation of the free edges.22 Finally, the dilated or distorted annulus is stabilized with an annuloplasty ring or band. The ultimate goals are to restore the normal height and shape of residual leaflet segments and the normal relationship of the surface areas of the anterior and posterior leaflets to the annular size in a given patient. To prevent systolic anterior motion (SAM) of the anterior mitral leaflet, it is important to keep the coaptation point of the leaflets from being too close to the ventricular septum and causing left ventricular outflow tract obstruction.22–24
To resolve SAM of the anterior leaflet intraoperatively, volume loading, increasing afterload, and stopping inotropic drugs may help. In some cases, repeat repair or replacement of the mitral valve with a prosthetic valve may be necessary. SAM has been reported in as many as 14% of patients with myxomatous disease after valve repair, but it affects fewer than 10%.25 The need for complex sliding annuloplasty or other reconstruction is reduced with the use of new large-diameter rings that accommodate larger leaflets and move the coaptation point farther away from the septum.26
Isolated anterior leaflet repair or concomitant repair of anterior and posterior leaflets traditionally has been considered more complex. However, the experiences and approaches to repair of the anterior leaflet continue to evolve and improve. The major challenge to anterior leaflet repair is created by the anterior portion of the mitral annulus sharing tissue with the aortic valve. This makes the anterior leaflet less amenable to the quadrangular resection because of increased rigidity. There are multiple approaches to anterior leaflet repair, including limited triangular resection of the anterior leaflet, chordal shortening and transposition, artificial chordal replacement, and edge-to-edge repair.27,28 However, there has not been a consensus or scientific evidence of a preferred approach. There is substantial variability from institution to institution in the approach to anterior leaflet repair and combined repair of the anterior and posterior leaflets.
Intraoperative 2DTEE and RT3DTEE should be routinely performed in mitral valve repair. Evaluation of the mitral leaflets by 3DTEE has been shown to be superior to 2DTEE, and the complexity of the repair can be predicted.17,29,30 When planning mitral valve repair, the following are important determinants of the complexity: (1) severity of mitral regurgitation, (2) the mechanism of mitral regurgitation, (3) the anatomic lesions, (4) the underlying degenerative etiology (Barlow disease vs. fibroelastic deficiency), (5) segmental leaflet pathology, (6) subvalvular pathology and calcification, (7) left ventricular size and function, (8) left atrial size and presence of thrombus, (9) other concomitant valve lesions, (10) presence of a patent foramen ovale, and (11) right ventricular function.15,31 Measurements, including the mitral annular diameter, anterior and posterior mitral leaflet dimensions, and the distance from the coaptation point of the mitral leaflets to the septum, can be made with intraoperative TEE to predict the likelihood of SAM developing after mitral valve repair.23 A short anterior mitral leaflet, a long posterior leaflet, or a short distance from the coaptation point to the septum is associated with higher likelihood of SAM developing after repair. The chance of SAM after repair can be minimized with knowledge of these echocardiographic parameters. After completion of the repair and removal of the patient from cardiopulmonary bypass, the valve should be reassessed for any residual mitral regurgitation, mitral gradient, and SAM. Ventricular function and any residual intracardiac air also can be assessed. A finding of more than mild mitral regurgitation should lead to consideration of reexploration of the mitral valve to identify the etiology of residual regurgitation, such as uncorrected prolapse, separation of a leaflet cleft, a defect in a leaflet-to-leaflet coaptation, or a perforation of a leaflet from a ring suture near the leaflet hinge. For persistent SAM with residual mitral regurgitation and a significant outflow tract gradient, reoperative correction should be considered. The main surgical approaches to address SAM are leaflet shortening or the use of a short neochordae to displace the posterior leaflet of excess leaflet height into the ventricle and out of the orifice.24 A larger mitral ring annuloplasty device can also be placed if there is a question about inappropriate sizing of the annulus during the initial repair.26
Patients who have undergone mitral valve repair should have a follow-up transthoracic echocardiography (TTE) prior to discharge to assess the mitral regurgitation under normal loading and pressure conditions. The presence of residual moderate or severe mitral regurgitation should lead to strong consideration of repeat valve exploration for additional repair or replacement. Quantification of mitral regurgitation can be assessed by RT3DTTE with 3D vena contracta methods.32
Data on Chordal Preservation
RT3DTEE has been shown to be superior to 2DTEE for assessment of chordal rupture.33,34 Although RT3DTEE has not yet been evaluated for surgical replacement of chordae, an emerging paradigm is based on the use of polytetrafluoroethylene (PTFE) neochordae to reconstruct the support of the free edge of prolapsing segments and to ensure a good leaflet coaptation surface, thereby preserving the valve without SAM of the anterior leaflet.35,36 A common technique is to anchor the neochordae into the papillary muscle heads in the fibrous portion and attach the branches to the free edges to prevent excess tension on the leaflet margins.37 With significant excess posterior leaflet height, the neochordae are sufficiently short to displace the prolapsing segment into the left ventricle to maintain a large surface of coaptation for the anterior leaflet while preventing SAM of the anterior leaflet. The surgery is significantly more complex with the placement of artificial chordae. On one hand, residual mitral valve prolapse and residual regurgitation may occur when chordae are too long. On the other hand, leaflet restriction and residual mitral regurgitation may occur when chordae are too short.
Chordal transfer to the free margin within the same segment or chordal transposition from one segment to another may be considered if residual prolapse remains after resection of abnormal tissue. In cases of mitral valve prolapse without redundant leaflet tissue, limited resection or artificial chordal replacement may be performed, followed by reinforcement with an annuloplasty ring or band. Diffuse mitral annular calcification can pose a special challenge to valve reconstruction.38 With the presence of calcification of the chordae, papillary muscles, leaflets, and annulus, careful debridement of calcified tissue often is required to restore normal leaflet motion and to ensure an adequate surface of coaptation.
Because the posterior aspect of the mitral annulus is not contiguous with the fibrous skeleton of the heart, pathologic annular dilation may result from chronic regurgitation associated with atrial and ventricular enlargement. Regardless of the leaflet and chordal techniques used, an annuloplasty ring or band often is necessary to restore the normal circumference and shape of the mitral valve to match the available leaflet tissue. The appropriate ring size for the amount of leaflet tissue is determined by the surface area of the anterior leaflet; a standard-length band has been used by some groups with excellent results.39 Absence of an annuloplasty at the time of mitral valve repair is one of the strongest predictors of repair failure, resulting in recurrent moderate or severe mitral regurgitation.40
Surgical Morbidity and Mortality of Mitral Valve Repair
Mitral valve repair is associated with an operative mortality rate of 3% or less.41–45 This figure is close to 1% in high-volume centers.46 The most common cause of death is heart failure. Predictors of death include advanced age; poor NYHA functional class; atrial fibrillation; low ejection fraction; high left ventricular end-systolic dimensions; and other comorbidities such as diabetes, obesity, renal disease, and chronic lung disease.41,47,48 In an analysis from the Society of Thoracic Surgeons National Adult Cardiac Surgery Database, major postoperative complications of mitral valve repair before discharge include prolonged (>24 hours) ventilatory support (7.3% of patients), renal failure (2.6%), and stroke (1.4%).48 Reoperation during initial hospitalization was reported in 6.3% of patients. Approximately 5% of patients have thromboembolism develop in the first 5 years after initial surgery.41,49 Intraoperative conversion to mitral valve replacement occurs in 2% to 10% of cases. Other rare complications of mitral valve repair include damage to important structures around the mitral apparatus such as the left circumflex coronary artery, the bundle of His, and the aortic valve.
Recurrent Mitral Regurgitation and Reoperation Rates
The most important late complication of mitral valve repair is recurrent mitral regurgitation. The recurrence rate is higher in patients with functional mitral regurgitation. Recent reports of recurrent moderate to severe mitral regurgitation range from 5% to 29%.40,50 Other reports cite recurrence of moderate to severe mitral regurgitation in 1% to 2% of patients per year during mid-term follow-up.40,51 The reoperation rate is very low in degenerative mitral valve surgery; 15-year freedom from reoperation is 95%.21 Reoperation to treat recurrent mitral regurgitation after primary repair is required in approximately 0.5% to 1.5% of patients per year.45,47 The most common cause of recurrent mitral regurgitation is progression of the underlying degenerative process of the original valve. Leaflet shortening or scarring, ring dehiscence, and infective endocarditis also may cause recurrent regurgitation. In patients with functional mitral regurgitation, conventional ring annuloplasty is prone to failure. Recurrent severe mitral regurgitation is reported in 25% of patients as early as 1 year after surgery.52 Other rare complications following mitral valve repair include pannus in-growth triggered by the mitral annuloplasty ring and hemolysis that may justify the need for reoperation.
Results of Mitral Valve Repair Versus Replacement
Mitral valve replacement can be performed with either a mechanical valve or a bioprosthetic valve. Mechanical valves, on one hand, are associated with the risk of thromboembolism and require lifelong anticoagulation. On the other hand, bioprosthetic valves are associated with the risk of valve deterioration and failure. There is also the risk of prosthetic valve endocarditis. If the chordae tendineae are severed during mitral valve replacement, the ventricular wall is no longer anchored to the valve apparatus, and the tethering effect of the chordae is lost. As a result, left ventricular wall stress increases and left ventricular function deteriorates in the long term.53–56
Successful mitral valve repair is achieved in approximately 90% of all surgical cases of chronic mitral regurgitation regardless of lesions or associated leaflet dysfunction.57 Mitral valve repair is preferable to replacement for most patients undergoing surgery for mitral regurgitation. The advantages of mitral valve repair include lower rates of thromboembolism, higher resistance to endocarditis, effective late durability (reportedly for as long as 25 years), and no need for anticoagulation in most patients.58,59 Repair has been demonstrated to be superior in the setting of combined coronary bypass procedure, reoperation, double-valve procedure, and in the older adult population.60–63 Mitral valve repair should be the standard of care whenever possible.
However, to date no prospective randomized trials have compared mitral valve repair with replacement. Studies reported thus far are not completely isolated from bias or heterogeneity in the cohort. Data from observational studies suggest a benefit of mitral valve repair.64–66 A meta-analysis compared mitral valve repair with replacement for degenerative mitral valve disease and suggested lower survival rates in mitral valve replacement compared with repair.64 Another report showed a significant 5-year survival benefit for patients who underwent mitral valve repair versus replacement, but no difference in quality of life.65 However, in a study of patients who underwent an isolated primary operation for degenerative mitral valve disease, there was no significant difference in survival at 5, 10, or 15 years between mitral valve repair versus replacement among propensity-matched cohorts.66
Individual and institutional experience is crucial in determining the likelihood of the success of a mitral valve repair. High-volume centers tend to have the lowest mortality rates and the greatest proportion of patients undergoing mitral valve repair rather than replacement.46 In contemporary practice, mitral valve replacement for degenerative disease as a primary operation should be infrequent. The most common scenario for a replacement should be an end-stage Barlow disease, typically seen in older adults with longstanding disease. If valve replacement is necessary, a chordal sparing procedure should be considered to preserve the annular-papillary continuity. Although calcified leaflet segments require resection, noncalcified segments with intact chordae can be incorporated into the annulus with sutures used to implant the prosthesis.
Advancements in Mitral Valve Repair
The principles of mitral valve repair have evolved since the classic Carpentier technique, which primarily involves quandrangular leaflet resection, transposition of normal chordae to other areas of prolapsing leaflet tissue, and placement of an annuloplasty ring or band.20 Because the mitral annulus is a dynamic, saddle-shaped structure, newer annuloplasty rings have increasingly attempted to replicate the natural shape and provide flexibility for the annulus. Optimal size and material properties of an annuloplasty ring are important because they change the geometry and dynamics of the mitral annulus after implantation and directly affect the durability of mitral valve repair. Annuloplasty rings with a disproportional reduction in the septal-lateral dimension are designed to restore leaflet coaptation in functional mitral regurgitation. Annuloplasty rings of different shapes and rigidity have been studied, as have their effects on anterior mitral leaflet and mitral annular dynamics in animal models, with the goal of improving long-term durability of the mitral valve repair.67
Recently, surgeons in selective centers have gained experience in minimally invasive approaches to mitral valve repair. Although minimally invasive surgery may broadly refer to avoidance of a full sternotomy, robotic approach to mitral valve repair is becoming increasingly popular, especially in patients who are young and asymptomatic. In a recent series of 261 consecutive cases of robotic mitral valve repair for posterior leaflet prolapse, no deaths were reported, although the cohort was skewed toward a low-risk group.68 In addition, the robotic approach to mitral valve repair has been limited to degenerative posterior leaflet prolapse, which has the most extensive experience, and overall outcomes depend greatly on the experience of the surgeons. Data comparing the quality of repair and outcomes of minimally invasive approach to conventional surgery are still insufficient. There is also growing experience with minimally invasive mitral valve repair performed through a right mini-thoracotomy. In a single-center series involving 1339 patients, the 30-day mortality rate was 2.4%, the 5-year survival rate was 82.6%, and the reoperation rate was 3.7%.69 These results are similar to those of traditional surgery. However, these minimally invasive approaches still require further evaluation with respect to widespread generalizability and cost effectiveness.
Edge-to-edge approximation of the leaflets (the Alfieri technique) has reemerged in traditional mitral valve repair.70,71 This technique typically is used with other repair techniques and annuloplasty because recurrent mitral regurgitation and a high risk for reoperation often result when this technique is used without an annuloplasty ring. One risk of edge-to-edge repair is restriction of the the mitral orifice, which could potentially lead to mitral stenosis if thickening or stiffening of the leaflets is present.
For complex ischemic mitral valve disease, some procedures have focused on the subannular procedures to approximate the papillary muscles, pull the papillary muscles toward the annulus to release leaflet tethering, or cut secondary chordae to reduce tethering of the leaflets.72 Most patients with mitral regurgitation undergoing coronary bypass are treated with a simple annuloplasty ring or band.
Percutaneous Approaches to Mitral Regurgitation
A variety of new percutaneous approaches to mitral valve disease are in early clinical use or undergoing preclinical investigation. Recent reviews of this broad area have been published.73,74 The edge-to-edge surgical technique has been modified for a percutaneous approach, such as the MitraClip (Abbott Vascular, Santa Clara, CA). This device can be applied on opposing prolapsed segments of the mitral leaflets as a practical approach for patients with a simple localized prolapsed segment such as A2, P2, or both. The pathology and mechanism of mitral regurgitation need to be thoroughly assessed to determine the suitability for repair with the device. TEE, especially RT3DTEE, is critical to guide device deployment so that an immediate assessment of the device stability and results of the repair can be made.
As in the case of deployment of clips to the leaflet edges, multiple TEE views are important to center the clips over the mitral valve orifice and align the clips with the leaflet edges. The clip is oriented perpendicular to the coaptation line, and simultaneous perpendicular planes can be obtained.75 The clip is closed when the leaflets have fallen into the clip arms. The degree of reduction of mitral regurgitation can be immediately assessed. If the initial results are suboptimal, the clip can be repositioned, or a second clip can be deployed. The mid-term results of this percutaneous approach have shown similar improvements in clinical outcomes but superior safety compared with traditional surgery, despite lower success at reducing mitral regurgitation. Of the patients with successful procedure, 77% had less than 2+ mitral regurgitation on discharge.76 In patients with severe symptomatic mitral regurgitation deemed to be at high surgical risk because of comorbidities, the MitraClip procedure has been shown to reduce severity of mitral regurgitation in the majority of patients, with improvement in clinical symptoms and left ventricular reverse remodeling at 1-year follow-up.77 However, this percutaneous approach does not address the shape of the dilated or distorted annulus without an accompanying annuloplasty. For surgical experience with the edge-to-edge technique not accompanied by an annuloplasty, 30% of patients require reoperation for significant recurrent mitral regurgitation at 5 years compared with 8% of those with an annuloplasty.70,78
There is significant interest in placing devices in the coronary sinus to push against the posterior mitral annulus to improve coaptation of the posterior and anterior mitral valve leaflets by reducing the overall circumference and the anteroposterior diameter of the mitral annulus. However, the coronary sinus does not lie directly adjacent to the posterior mitral annulus and often is not in the same plane as the annulus.79 Given the variability of the anatomy and position of the coronary sinus and the great cardiac vein, the dimensions of the coronary sinus from both the mitral annulus and the center of the mitral valve orifice need to be accurately determined.80 The position of the cardiac veins in relation to the mitral annulus may predict the procedural success and also the course of the left circumflex artery, whose branches need to be assessed prior to the deployment of the device. With the left circumflex coronary artery or its branches lying between the mitral annulus and the coronary sinus, placement of the device may compromise the left circumflex artery.81 Although cardiac computed tomography and magnetic resonance imaging can provide such information prior to the procedure, it is prudent to use RT3DTEE as a guide with regard to the anatomic landmarks during the procedure and to assess the immediate results after deployment of the devices.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree