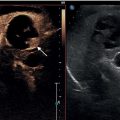
Matteo Rosselli1,2 and Robert de Knegt3 1 Department of Internal Medicine, San Giuseppe Hospital, USL Toscana Centro, Empoli, Italy 2 Division of Medicine, Institute for Liver and Digestive Health, University College London, Royal Free Hospital, London, UK 3 Department of Gastroenterology and Hepatology, Erasmus MC University Medical Centre, Rotterdam, The Netherlands Ultrasound is an imaging technique that has the great benefit of providing a dynamic image of anatomy, physiology, and pathology in real time. The extension of physical examination by directly correlating images with a patient’s signs and symptoms [1] is called echoscopia, meaning literally ‘to look into with the use of ultrasound’ (Figure 12.1). This medical approach, known as point‐of‐care ultrasound (POCUS), was employed initially to detect free intraperitoneal fluid in multitrauma patients (focused assessment sonography of trauma or FAST) [2, 3]. With time POCUS has developed, proving to be a life‐saving medical tool for clinicians through the stages of diagnosis, resuscitation, surgery, and post‐operative critical care [4]. However, although it finds immediate applications in specialties such as emergency, acute, and intensive care medicine, it is clear that many medical fields can benefit from its use [1]. This chapter describes the technical aspects and practicalities of liver POCUS examination and its use in different clinical scenarios, such as in acute presentations of liver pathology, rapid assessment of chronic liver disease (CLD), post‐procedural findings, and finally an overview of the importance of POCUS in the critically ill patient, highlighting the differences that might be found in cirrhosis. In this chapter a lot of emphasis has been given to the ultrasound assessment of haemodynamic instability and respiratory failure, since both can be a cause and consequence of severe liver dysfunction and are thus important sequelae and findings to recognise and assimilate with the whole patient clinical scenario. POCUS should provide information on the anatomical site that is the object of a clinical inquiry, as well as on the interactions between organs that are anatomically close and physiologically linked. It is a quick, problem‐solving assessment tool for improving patient management in a timely manner. POCUS does not differ from ‘regular’ ultrasound, although it is more goal directed and driven by the most likely clinically diagnosis. As such, training for ‘regular’ and ‘point‐of‐care’ ultrasound should not be different. Most clinicians using POCUS as part of ‘regular’ sonography will have had full training according to the guidelines of national or international ultrasound societies. Changes in epidemiology or the occurrence of new diseases might attract those with ‘regular’ ultrasound experience to opt for POCUS or focused ultrasound. An important example today is the increasing use of ultrasound in patients with Covid‐19 pulmonary disease [5]. However, at the present time scientific or professional societies do not offer POCUS training, since it is considered always embedded in general sonography. Selective POCUS training is currently only being offered by commercial institutions and mostly lacks accreditation, and it seems that those with POCUS experience only might be valuable in screening situations or when searching for a specific finding (e.g. screening for aortic aneurysms, free fluid in trauma patients, biliary obstruction in patients with jaundice), whereas in the clinical setting full training is to be preferred. Figure 12.1 The ultrasound beam is able to penetrate by ‘looking through’ the superficial tissues, revealing deeper structures, organs, and eventually underlying pathological findings in real time. The general scanning technique should follow the standard operating procedure used to image the hepato‐biliary system, the spleen, and the splanchnic circulation (Chapter 3). What changes is the approach and the clinical and physiopathological integration that comes with POCUS, including the haemodynamic as well as respiratory assessment, especially useful in clinically unstable patients. With regard to haemodynamic assessment, cardio‐hepatic interactions are a cornerstone of POCUS assessment. This is evident if one considers that the inferior vena cava (IVC) before terminating in the right atrium crosses the liver, receiving the blood drained from the hepatic veins. The examination technique requires the use of a curvilinear transducer or phase array transducer (usually used by cardiologists or intensive care physicians) that is placed just below the xiphoid process with a sagittal orientation, in order to obtain a longitudinal view of the left liver lobe and the retrohepatic IVC. Measurements are performed in B‐mode or M‐mode, approximately 2–3 cm from the atrio‐caval junction. For completion a transverse subcostal approach will ensure visualisation of the hepatic veins draining into the IVC, and by tilting the probe upwards a more panoramic four‐chamber view of the heart can be obtained. This approach is particularly useful when right heart dysfunction and a pericardial effusion need to be ruled out. In case of overlying bowel gas or when a certain pressure needs to be exerted with the risk of external IVC compression, it is preferable to use a coronal transhepatic approach by scanning the patient intercostally along the mid‐axillary line (Figure 12.2) [6]. In normal individuals the absolute anteroposterior diameter of the IVC is 1.5–2.5 cm. During spontaneous breathing the IVC collapses in inspiration, because the negative intrathoracic pressure favours venous return [7]. The calibre and the respiratory collapsibility of the IVC have been shown to correlate with right atrium pressure and hence central venous pressure (CVP). As a general rule, an inspiratory collapse ≤50% of the maximal expiratory diameter is physiological; an IVC collapse at end inspiration >50%, especially in the case of normal or narrow IVC, correlates with low CVP; while a collapse <50% in the case of a wide IVC is associated with high atrial pressure and hence increased CVP (Figure 12.3) (Video 12.1) [6]. A detailed description of lung ultrasound scanning technique is beyond the scope of this text, which will rather refer to the interpretation of different sonographic patterns and clinical scenarios related to respiratory failure. The air–fluid interaction between the pleura and the lung parenchyma provides the necessary information to differentiate between pleural effusion, pulmonary oedema, interstitial as well as lobar pneumonia, and other pathological findings [8]. It is important to highlight that in order to assess lung pathology sonographically, at least part of the process causing respiratory failure must be in contact with the pleura. In fact, the interposition of ventilated lung between the ultrasound beam and an area of consolidation, for example, will cause an acoustic barrier, giving a false‐negative result or underestimating the severity of an underlying condition. This is surely a limitation of lung ultrasound that should be kept in mind. Among the different findings of lung ultrasound the one we wish to highlight is related to B‐lines as an expression of fluid that replaces air within the lung interstitium and eventually alveoli that can be an expression of fluid overload, but also inflammatory changes. B‐lines are related to long laser‐like hyperechoic lines that arise from the pleura and move sincroniously with the lung. The more fluid is accumulated withing the lung, regardless of its cause, the more confluent the B‐lines will be. In general, the assessment of a patient with respiratory failure should always include both lung fields, since monolateral and bilateral findings can have different pathological and diagnostic implications. In addition, the IVC calibre and its collapsibility should also be assessed, since the interpretation of ‘cardio–respiratory’ interaction has a high accuracy in distinguishing respiratory failure with high CVP from respiratory failure with low CVP, which has a completely different physiopathological background and clinical management. In this respect the differential diagnosis between cardiogenic pulmonary oedema and respiratory distress syndrome seen in sepsis is an excellent example of the importance of POCUS clinical application. Figure 12.2 The retrohepatic inferior vena cava (IVC) crosses the liver, receiving the blood from the hepatic veins before terminating in the right atrium. The IVC can be visualised using a sagittal (a) or subcostal approach (b). The subcostal approach grants a more panoramic view of the three hepatic veins draining into the IVC. In addition, by tilting the probe upwards the IVC can be seen draining into the right atrium. In case of overlying bowel gas or other technical limitations, the IVC can be imaged by using an intercostal coronal approach (c). LHV, left hepatic vein; MHV, medium hepatic vein; RHV, right hepatic vein; RA, right atrium. Figure 12.3 Although constitutional variability has been described, in normal conditions during respiration the inferior vena cava (IVC) has a diameter of approximately 1.5–2.5 cm, and its collapsibility is approximately 50%. A calibre <1.5 cm with a collapsibility >50% is associated with hypovolemia and low central venous pressure (CVP); a diameter >2.5 cm with a collapsibility <50% is an expression of increased CVP. Four pairs of images are explicatory examples. The left image of each pair represents the retrohepatic IVC calibre at the end of expiration, the right image the retrohepatic IVC calibre at the end of inspiration. (a) The IVC has a normal calibre of 20 mm and at end inspiration it is 11 mm, therefore showing a reduction in calibre of approximately 50%. (b) A similar case is shown where a normal IVC calibre is initially observed, with subsequent significant calibre reduction during inspiration. (c) The IVC is almost collapsed in both respiratory phases as a sign of severe hypovolemia. (d) The IVC is distended in both respiratory phases with no modification of its calibre during inspiration, in keeping with increased CVP that in this case was secondary to severe pulmonary hypertension (Video 12.1). Figure 12.4 During inspiration the diaphragm lowers, allowing lung expansion. On ultrasound the normal ventilated lung parenchyma corresponds to a ‘lung curtain’ that dynamically fills the costophrenic recess during respiration. The white arrows point to the interface between the expanding ventilated lung and the liver (a) and spleen (b) parenchyma (Video 12.2). Figure 12.5 (a) Right pleural effusion (arrow) with complete atelectasis of the right lung base (asterisk). (b) Large left pleural effusion (arrow) with complete left lung collapse (asterisk). (c) Subcostal transverse view showing bilateral pleural effusion (arrows) and left lung base atelectasis (asterisk). Figure 12.6 (a) Oblique transverse subcostal view showing a large right pleural effusion (asterisk), right and left atrium and liver. (b) Intercostal approach revealing septations within the effusion that are further confirmed at higher magnification with a linear transducer (c). Features in keeping with multiloculated pleural effusion caused by a Streptococcus pneumoniae infection. RA, right atrium; LA, left atrium. Figure 12.7 Subcostal transverse view revealing large echogenic right pleural effusion (asterisk). A 14 F pigtail drain was positioned (arrow), with progressive drainage of what appeared to be a large empyema. The sequence of images shows reduction of the effusion and decompression of the right liver lobe. Figure 12.8 Patient with respiratory failure and sepsis. Point‐of‐care ultrasound reveals severe hypovolemia as demonstrated by a threaded IVC with complete collapse during inspiration (a, left side image). There are features of diffuse right lung hepatisation (a, middle image). Note is made of air bronchogram (a, right side image, white arrows) and interstitial involvement of the ventilated parenchyma (a, right side image, red arrows). There is evidence of air‐fluid filled cavity within the lung compatible with lung abscess (b) (Video 12.4). Figure 12.9 (a) Bilateral B‐lines and sub‐pleuric lung consolidation (arrow). (b) There is threading of the inferior vena cava (arrow) with complete collapse during inspiration. Findings in keeping with interstitial pneumonia and severe hypovolemia. Figure 12.10 A mixed alveolar‐interstitial pattern in a patient with severe fungal pneumonia. There is a large sub‐pleuric round parenchymal consolidation (asterisk) surrounded by diffuse interstitial lung involvement (arrows). It is important to bear in mind that one or more physiopathological conditions might co‐exist, sometimes making ultrasound findings difficult to interpret unless appropriate integration of all the necessary information and organ interactions is considered. Since the change in size of the IVC depends on intrathoracic pressure variations, which occur during respiration, the magnitude of the respiratory effort represents a crucial but not quantifiable variable [9]. Regardless of the volaemic state, the respiratory effort might lead to collapse of the IVC in conditions of euvolaemia, while patients with superficial respiration might have poor IVC collapsibility even in cases of hypovolaemia. Increased CVP as an expression of pulmonary hypertension is often found in chronic obstructive pulmonary disorder (COPD) and asthma, two conditions that are characterised by positive intrathoracic pressure, especially during expiratory exertion, making IVC‐volaemic assessment not always reliable (see Video 12.6). It is of note that during positive pressure ventilation the respiratory shifts of the IVC will be the opposite to those in spontaneous breathing. Right‐sided heart failure, severe tricuspid regurgitation, pulmonary hypertension, cardiac tamponade, and constrictive pericarditis lead to IVC distension and thus a challenging haemodynamic/volaemic status assessment (Figures 12.11 and 12.12) (Videos 12.7–12.9). Table 12.1 summarises the sonographic findings of POCUS assessment in respiratory failure. Haemodynamic interactions are also considered. Figure 12.11 (a) Patient with a large right pleural effusion (asterisk) and tamponading pericardial effusion (white arrow). M‐mode section of the inferior vena cava (IVC) shows no respiratory variation in keeping with increased central venous pressure (CVP). (b) Point‐of‐care ultrasound assessment after pericardial and pleural effusion drainage. There is persistent distension of the hepatic veins, the IVC is also distended at the end of expiration; however, it almost completely collapses during inspiration in keeping with significant decreased CVP. M‐mode highlights the dynamic changes of the IVC calibre during respiration (red arrows). The arrow in (a) shows the pericardial space occupied by the effusion and collapse of the right heart cavities; the white arrow in (b) shows heart re‐expansion after drainage of the pericardial effusion (Video 12.7). Figure 12.12 An example of the interference of confounding factors and the usefulness of extending point‐of‐care ultrasound examination in a patient with severe respiratory failure and sepsis. (a) Lung ultrasound shows bilateral thick and confluent B‐lines (‘white lung’). (b) At higher magnification with a linear transducer, there is evidence of interstitial consolidation (arrowheads) and skip areas of ventilated lung (arrow). (c) The inferior vena cava (IVC) assessed intercostally is distended, with a very small inspiratory shift suggesting increased central venous pressure, although the patient was hypotensive. Usually a lung ultrasound showing these features is compatible with septic interstitial involvement, but the IVC is usually reduced in calibre. (d) For completion, a Doppler ultrasound scan of the lower limbs was performed showing bilateral deep vein thrombosis of the femoral veins (arrow). The white arrow points to a thrombus at the level of the bifurcation of the femoral vein. A computed tomography angiogram revealed bilateral pulmonary thromboembolism (Video 12.9). Obesity, ileus, and tense ascites are causes of technical, constitutional, and physiopathological interference. Since the IVC is a retroperitoneal structure, in case of abdominal distension the operator might need to exert significant pressure, potentially influencing it’s calibre; moreover, ileus might be associated with air‐filled distended bowel loops with acoustic shadowing. Therefore under these conditions an intercostal coronal approach is recommended. However, it should be kept in mind that elevated intra‐abdominal pressure leads to a complete loss of the relationship between IVC size, collapsibility, and fluid responsiveness. An important example can be seen in cirrhosis with tense ascites. In this case the IVC can have a variable calibre and can even be quite narrow, since often these patients are relatively hypovolaemic. However, minimal or no‐calibre variations are seen during respiration due to both increased intra‐abdominal pressure and reduced thoracic expansion secondary to diaphragmatic splinting (Figure 12.13) (Video 12.10) [10, 11]. In other circumstances upstream dilatation can be observed in IVC stenosis due to structural vascular or parenchymal abnormalities (Figure 12.14), or after interventional procedures such as in liver transplant, where the stenosis is usually at the site of the anastomosis. It is also possible that extrinsic IVC compression occurs, secondary to oedema of the graft, fluid collections, haematomas, or abscesses (Figure 12.15). In all these cases IVC calibre and collapsibility will not be a reliable index of CVP. Table 12.1 Different causes of respiratory failure with lung sonographic pattern and inferior vena cava (IVC) appearance.
12
Point‐of‐Care Ultrasound in Liver Disease
Basics of POCUS and Training Requirements

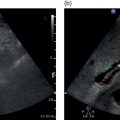
POCUS Examination Technique
![]()
![]()
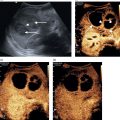
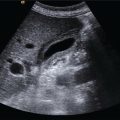
![]() As a basic ultrasound scanning approach, basolateral lung fields can be evaluated by imaging the costophrenic recess, which is found by following the upper‐postero‐lateral margins of both liver and spleen while the patient is lying in supine or lateral position. In normal conditions this space is dynamically covered by the ‘lung curtain’ related to the expansion of air‐filled lung parenchyma (Figure 12.4) (Video 12.2). In the presence of pleural effusion, the fluid fills the costophrenic space and the lung parenchyma is compressed and displaced proportionally to the amount and nature of the effusion itself (Figures 12.5–12.7) (Video 12.3). To evaluate the extension of the pleural effusion as well as other findings such as lobar pneumonia, atelectasis, or interstitial involvement, the operator should continue scanning upwards until the higher postero‐lateral lung fields are assessed. Although the posterior lung fields are better evaluated with the patient sitting up or in lateral position, an overdiaphragmatic lung consolidation/effusion can also be visualised by using a subcostal approach while the patient is supine. Due to the liver’s larger size, this approach usually favours right‐sided findings (Figures 12.6 and 12.7). The anterior lung fields should also be evaluated, especially in cases of pulmonary cardiogenic oedema, adult respiratory distress syndrome (ARDS), and suspected pneumothorax. Features of pneumonia include hepatisation of lung parenchyma in case of alveolar lobar pneumonia (Figure 12.8) (Video 12.4), while interstitial pneumonia is usually visualised as a combination of diffuse bilateral B‐lines in conjunction with pleural irregularities and clear hypoechoic sub‐pleuric areas of different sizes (Figure 12.9) (Video 12.5). A mixed pattern is characterised by signs of consolidation and diffuse thick B‐lines, usually adjacent to the areas of alveolar involvement (Figure 12.10). Of note is that the sonographic appearance of lung disease, especially in acute processes, may vary considerably during its development as well as in response to treatment, and this is why POCUS is such a useful tool due to its repeatability, cost‐effectiveness, and no radiation exposure.
As a basic ultrasound scanning approach, basolateral lung fields can be evaluated by imaging the costophrenic recess, which is found by following the upper‐postero‐lateral margins of both liver and spleen while the patient is lying in supine or lateral position. In normal conditions this space is dynamically covered by the ‘lung curtain’ related to the expansion of air‐filled lung parenchyma (Figure 12.4) (Video 12.2). In the presence of pleural effusion, the fluid fills the costophrenic space and the lung parenchyma is compressed and displaced proportionally to the amount and nature of the effusion itself (Figures 12.5–12.7) (Video 12.3). To evaluate the extension of the pleural effusion as well as other findings such as lobar pneumonia, atelectasis, or interstitial involvement, the operator should continue scanning upwards until the higher postero‐lateral lung fields are assessed. Although the posterior lung fields are better evaluated with the patient sitting up or in lateral position, an overdiaphragmatic lung consolidation/effusion can also be visualised by using a subcostal approach while the patient is supine. Due to the liver’s larger size, this approach usually favours right‐sided findings (Figures 12.6 and 12.7). The anterior lung fields should also be evaluated, especially in cases of pulmonary cardiogenic oedema, adult respiratory distress syndrome (ARDS), and suspected pneumothorax. Features of pneumonia include hepatisation of lung parenchyma in case of alveolar lobar pneumonia (Figure 12.8) (Video 12.4), while interstitial pneumonia is usually visualised as a combination of diffuse bilateral B‐lines in conjunction with pleural irregularities and clear hypoechoic sub‐pleuric areas of different sizes (Figure 12.9) (Video 12.5). A mixed pattern is characterised by signs of consolidation and diffuse thick B‐lines, usually adjacent to the areas of alveolar involvement (Figure 12.10). Of note is that the sonographic appearance of lung disease, especially in acute processes, may vary considerably during its development as well as in response to treatment, and this is why POCUS is such a useful tool due to its repeatability, cost‐effectiveness, and no radiation exposure. ![]()
![]()












Confounding Factors and Co‐existing Pathologies
Thoracic/Respiratory Factors
![]()
Cardiac Factors
![]()




Abdominal Factors
![]()
Respiratory failure
Ultrasound findings
IVC pattern
Cardiogenic pulmonary oedema
Bilateral lung diffuse continuous B‐lines
Distended IVC
Reduced IVC collapsibility
Adult respiratory distress syndrome (ARDS)/sepsis
Discontinuous bilateral B‐lines with signs of scattered lung consolidation with interstitial involvement
Ultrasound findings of septic source (e.g. liver abscess, infective colitis, pyelonephritis, pneumonia)
Collapsed or narrowed IVC
Lobar pneumonia
Monolateral or bilateral lung consolidation with air bronchogram
Usually narrowed IVC, unless pre‐existing pulmonary hypertension or co‐existing pathologies that increase central venous pressure (CVP)
Interstitial pneumonia
Usually bilateral B‐lines with irregular distribution and sub‐pleuric lung consolidations
IVC calibre varies according to the presence of sepsis, hypovolaemia, or other underlying causes that interfere, increasing or decreasing CVP
Pulmonary embolism
Negative lung ultrasound or monolateral or bilateral small wedged sub‐pleuric hypoechoic areas or larger atelectasic lung areas surrounded by effusion
Ultrasound findings of deep vein thrombosis have a high diagnostic positive predictive value for pulmonary embolism in case of suspicious clinical presentation and respiratory failure
Distended IVC as an expression of pulmonary hypertension
Pneumothorax
M‐mode barcode sign, in the majority of cases monolateral
IVC calibre varies according to the extension of pneumothorax, respiratory rate, hypovolaemia, the presence of tension pneumothorax with reduced venous return, and increased CVP ![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree

 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access