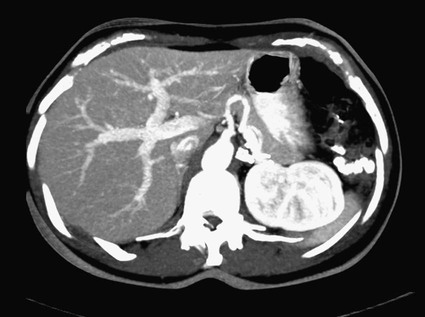
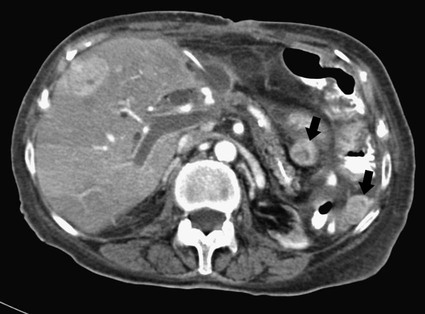
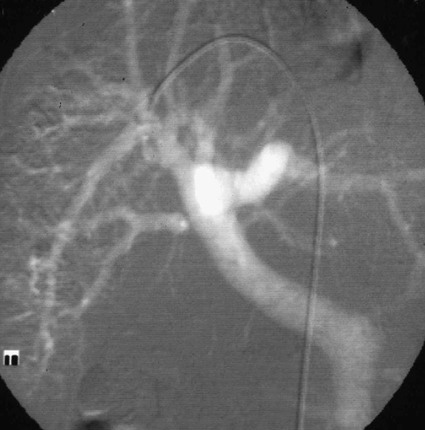
José I. Bilbao, Pablo D. Domínguez, Isabel Vivas and Antonio Martínez-Cuesta The importance of portal vein thrombosis (PVT) lies mainly in the complications of prehepatic portal hypertension, which in the chronic state causes bleeding through varices. Acute PVT is the main cause of prehepatic portal hypertension in the Western world1 and the primary cause of portal hypertension of any type in noncirrhotic patients in developed countries. PVT accounts for some 8% to 10% of all cases of portal hypertension. At present, causative factors are detected in most cases, although 8% to 20% are still considered idiopathic.2,3 In children, up to 50% of cases of PVT are secondary to abdominal infection (chiefly omphalitis), followed by congenital abnormalities of the portal vein (20%), which are generally associated with cardiac and biliary abnormalities (e.g., hypoplasia of the portal vein associated with biliary atresia).4 In adults, PVT is basically associated with cirrhosis, and its incidence increases as the disease progresses. The prevalence in patients with cirrhosis and hepatocarcinoma is as high as 44%.3 Other frequent causes in adults are neoplasm other than hepatocarcinoma, coagulation disorders, and inflammatory-infectious abdominal causes.2,3,5 When the cause is infectious, septic phlebitis of the portal vein associated with thrombosis may occur, and the process is known as pylephlebitis. Even when local causes of PVT are present, the existence of a prothrombotic predisposition or a latent myeloproliferative disorder should always be ruled out because these are frequent causes of PVT that respond to treatment.3,6 The acute and chronic forms of PVT are differentiated by the length of time over which they develop. The dividing line between the two has not been defined, and the criteria vary from author to author. Some consider cases diagnosed less than 40 to 60 days from the onset of symptoms to be acute3,7 in the absence of radiologic findings or complications related to chronic PVT. Others hold that acute cases in noncirrhotic patients must meet three conditions: (1) recent onset of symptoms, (2) no signs of chronic portal hypertension, and (3) absence of portoportal collaterals as shown by computed tomography (CT) or color Doppler ultrasound.8,9 The chronic form is diagnosed more frequently because PVT hardly ever produces symptoms or liver function disorders in the acute stage. The absence of acute clinical manifestations is mainly because in most cases, the PVT is not complete and is associated with rapid development of portoportal collaterals (cavernomatosis) and an increase in hepatic arterial blood supply.5,10 Consequently, diagnosis in these cases is made in the chronic phase when complications resulting from chronic portal hypertension appear (chiefly variceal bleeding) or abdominal images are taken for some other reason. At the acute stage, symptoms are generally nonspecific. If the thrombus extends as far as the distal mesenteric branches, it may cause ischemia or infarction of the mesenteric veins, which produces abdominal pain, nausea, vomiting, and slight ascites. The pain is characteristically disproportionate to the findings on clinical exploration of the abdomen.11 The existence of peritoneal irritation and ascites indicates necrosis of the wall and perforation. In such cases the prognosis is poor,3,11 and urgent surgery is required, with 13% to 50% mortality being reported.7 In cases of pylephlebitis, fever, leukocytosis, and abdominal pain are also present. In the chronic form, complications proper to portal hypertension occur, principally bleeding through varices, splenomegaly, and hypersplenism. The varices are most commonly esophageal (90%-95%), followed by anorectal (80%-90%) and gastric (35%-40%).4 Portal hypertensive gastropathy is rare in the absence of cirrhosis. In cirrhotic patients, portal hypertension and preexisting poor liver function make PVT and bleeding varices a serious complication that is poorly tolerated.4 PVT also renders liver transplantation difficult.12 In contrast, portal cavernomatosis frequently causes secondary biliary disorders, mainly stenosis and dilation, known by the generic name of portal biliopathy. However, only a small proportion of patients with cavernomatosis have clinical manifestations in the form of jaundice, cholangitis, choledocholithiasis, or cholecystitis.4 In children, the clinical findings are similar, except that growth is also retarded for reasons not fully understood.4 The prognosis for patients with PVT is determined by the pathologic conditions that cause it and, in acute cases, by the degree of mesenteric involvement. The prognosis is good in the absence of malignancy, cirrhosis, sepsis, or thrombosis of the mesenteric vein.3,5 Bleeding from varices is the principal complication of PVT, but recent studies have observed no significant changes in survival of these patients as a result of variceal bleeding at diagnosis in the absence of preexisting hepatic pathology,3 probably because of the efficacy of endoscopic treatment. However, in patients with bleeding from digestive varices in the presence of cirrhosis, mortality is much higher (30%-70% vs. 1%-5%).2,13 In cases of suppurative pylephlebitis, the prognosis is poor, and mortality may be as high as 80%.6 Four anatomic categories related to the extent of PVT3 have been defined and have clinical relevance for both prognosis and treatment: grade I, thrombus limited to the portal vein that does not reach the splenomesenteric confluence; grade II, thrombus extending to the superior mesenteric vein but with free mesenteric vessels; grade III, thrombus spreading diffusely through the splanchnic venous system but with the presence of large collaterals; and grade IV, thrombus spreading diffusely through the venous system but without large collaterals. The extent of thrombosis in mesenteric venous branches (grade III-IV) raises the risk for intestinal infarction and consequently the risk for death.3,14 The main portal vein is formed by the confluence of the splenic and superior mesenteric veins, and in the absence of anatomic variants, it divides at the porta hepatis into a right and left branch, which lead, respectively, to the right and left hepatic lobes. The left branch is divided into branches for each segment, whereas the right branch is divided into an anterior right branch for segments V and VIII and a posterior branch for segments VI and VII. This normal distribution is present in 65% to 90% of individuals.15,16 Anatomic variants usually occur in the right branch, and the most frequent ones are portal trifurcation (absence of the common right branch with direct division of the main portal vein into a left branch and two right branches [Fig. 103-1]), an anterior or posterior right branch originating from the left branch, and a posterior right branch originating from the main portal vein.11,15,16 The presence of one such variant can have considerable repercussions on surgery and interventional procedures.15 Color Doppler ultrasound is an effective technique for assessing PVT, and it is also fast, economical, and safe. This technique is generally used for detecting and monitoring PVT.2,8 If ultrasound brings the presence of PVT to light, the investigation must be completed with another technique (CT or magnetic resonance imaging [MRI]) to more accurately evaluate how far it extends into the mesenteric area and detect the presence of signs of intestinal ischemia or abdominal causes of PVT. At the acute stage, the thrombus may be markedly hypoechoic and pass unnoticed if color Doppler ultrasound is not performed. This study will show an area of absence of flow inside the lumen of the portal vein, with high sensitivity and specificity.4 Occasionally if portal flow is very slow, it may not be detected in the color Doppler study and might be confused with thrombosis. In such cases, pulsed Doppler imaging may reveal the existence of continuous slow flow.5,8 Color Doppler ultrasound also enables the specialist to assess whether arterial flow is present inside the echogenic thrombi. The presence of such arterial flow is highly specific for tumor thrombosis, although its sensitivity is variable.8 Regarding the use of intravenous contrast material in ultrasound, several studies have shown better sensitivity in the detection of PVT17,18 and in the characterization of tumor thrombi17 than with conventional and color Doppler ultrasound. Ultrasound assessment may be limited in obese patients, in patients with small livers, or if intestinal gas is present (this affects mainly assessment of the mesenteric branches).2 Many authors consider this study to be the technique of choice,11,14,19 particularly if multislice CT is available. Images obtained with CT offer the advantage of great anatomic detail, which makes them useful for evaluating intraluminal and extraluminal abnormalities, calcification of the portal vein intima, mural thrombosis, existence and distribution of portosystemic collaterals, and any mesenteric edema. Disadvantages include the use of radiation and intravenous iodinated contrast material. In general, a nonenhanced phase is performed first, followed by injection of 125 to 150 mL of intravenous contrast material at 3 to 5 mL/sec to obtain an arterial and a venous phase (portal, with a delay of 55-70 seconds from the beginning of injection of the contrast material).11 The initial phase without contrast medium makes it possible to detect very recent thrombi, which are hyperdense, as well as calcifications in the intima of the portal vein, and allows analysis of whether the thrombus enhances after the administration of contrast medium.1 After injection of intravenous iodinated contrast medium, the thrombus appears as a persistently hypodense area in the lumen of the portal vein, which is generally associated with peripheral enhancement corresponding to the vein wall (Fig. 103-2).11,14 If the thrombus is due to a tumor, it may enhance after administration of contrast medium, especially in the arterial phase.1 Conversely, the coexistence of hypodensity in the superior mesenteric vein, thickening of the wall of bowel loops, and ascites indicates transmural intestinal infarction, and urgent surgery may be necessary.11 Another common finding is the presence of heterogeneity in enhancement of the hepatic parenchyma. In general, clearly defined areas of hypodensity are observed and attributable to alterations caused by an irregular decrease in the uptake of contrast medium or the glycogen or fat content. On other occasions, areas of greater enhancement can be seen in the arterial phase because the arterial blood that supplies these areas of decreased portal flow increases more markedly.1 Magnetic resonance angiography (MRA) is an excellent technique for detecting PVT; its very high sensitivity and specificity (100% and 98%, respectively)20 are greater than those of CT and color Doppler ultrasound and similar to those of invasive techniques.20 It involves no ionizing radiation, the intravenous contrast material used is less nephrotoxic, and only in exceptional cases does it trigger allergic reactions. In comparison to CT, spatial resolution is lower, and the technique is less sensitive in detecting calcifications of the portal intima. It may also overestimate the degree of stenosis because the signal is degraded by turbulence, and it is not able to assess the lumen of metal stents. Moreover, much longer exploration time is needed (30-60 minutes), it is more expensive, and in general it is less widely available. To assess the portal system, MRA uses ultrafast three-dimensional sequences with digital subtraction angiography (DSA) after administration of paramagnetic intravenous contrast material. The sequences are obtained in the venous phase or in early equilibrium. It offers excellent visualization of the vascular lumen but poor spatial resolution of other structures and should therefore be complemented with conventional spin-echo or gradient-echo sequences.11 MRA without intravenous contrast material but with time-of-flight or phase-contrast sequences is less sensitive, although the latter can be used to evaluate the direction and speed of flow.11 MRA has been compared with DSA, and no significant differences were found in the detection of PVT.20 In conventional spin-echo and T1-weighted gradient-echo MR sequences, the thrombus is usually hyperintense when it has been developing for less than 6 weeks and isointense in chronic cases. On T2-weighted sequences, it is generally hyperintense.21 Invasive techniques are reserved for cases in which other techniques prove inconclusive or a percutaneous therapeutic procedure is to be performed.4,6,11 Such techniques are limited by the very fact of being invasive, as well as because they depend on flow dynamics and use intravenous iodinated contrast material and ionizing radiation. Indirect portography consists of assessing the venous return time of an injection of contrast medium into the superior mesenteric artery (SMA) or splenic artery. It is the most sensitive procedure for assessing thrombosis in the small mesenteric veins.19 Signs of thrombosis include the presence of defects in intraluminal repletion, delay in visualization of the venous system, secondary spasm of the mesenteric arteries, and prolonged opacification of the mesenteric arterial arcades.14 If PVT has developed in the chronic form, portosystemic collaterals will exist and can limit the extent to which the portal tree can be visualized if the contrast medium escapes through them in a hepatofugal direction. In direct portography, iodinated contrast medium is directly injected into branches of the portal vein via a transhepatic or transjugular route or through a patent umbilical vein. Less frequently, the transsplenic route or a mesenteric vein after minilaparotomy is used.22 Direct access to the portal vein has the advantage that the images are not limited by the existence of collaterals with hepatofugal flow, and pressure can be measured and therapeutic procedures carried out. Nonetheless, there is a risk of bleeding through the puncture point, especially if it is transhepatic, and a slight risk of thrombosis or infection of the portal vein also exists.21 In this technique, CO2 is injected through a catheter with a balloon wedged in a hepatic vein, generally the middle or right vein. The CO2 spreads backward through the portal tree and acts as a negative contrast agent (Fig. 103-3).23,24 Only one venous puncture is necessary (femoral or jugular), and it is possible to measure free and wedged hepatic pressure and even perform transvenous hepatic biopsy through the same access. CO2 is safer than iodinated contrast medium for performing this wedged injection because its lower viscosity means the possibility of capsular rupture due to high pressure is almost ruled out. Moreover, there are no allergic reactions or nephrotoxic effects, cost is low, there is no limit to the dose, and it spreads more easily through smaller vessels, making it possible to more accurately visualize the portal tree.23,24 In comparison to direct transjugular portography and indirect arterial portography, this method has been shown to be a safe and effective technique for assessing the portal vein.23,24 In cases of widespread PVT, it is the best technique for assessing the intrahepatic portal branches.23 Its drawback is that it does not show portosystemic collaterals as well as other techniques.
Portal-Mesenteric Venous Thrombosis
Etiology
Clinical Presentation
Prognosis
Imaging
Portal Vein Anatomy
Imaging Techniques
Color Doppler Ultrasound
Contrast-Enhanced Computed Tomography
Magnetic Resonance Imaging
Invasive Techniques
Indirect Portography
Direct Portography
Carbon Dioxide Wedged Hepatic Venography
Radiology Key
Fastest Radiology Insight Engine