Technical Aspects
Positron emission tomography and computed tomography (PET/CT) represents the successful technical combination of multidetector computed tomography (MDCT) and PET into a single scanner. PET with the fluorine-18 (18F)–labeled glucose analog fluorodeoxyglucose (FDG) provides metabolic imaging of tissues, both normal and diseased. FDG-PET provides valuable qualitative and quantitative metabolic information for both diagnosis and management. PET has been shown to be of value in diagnosing and staging malignant tumors as well as in follow-up oncologic imaging after surgical, radiation, or chemotherapeutic treatment. However, the sensitivity of PET in detecting hypermetabolic foci is compromised in large part by the low background FDG uptake when attempting accurate anatomic localization.
CT provides rapidly acquired data sets of high spatial resolution giving multiplanar information regarding the morphologic features and attenuation values of both normal anatomy and pathologic lesions. A major weakness of CT, however, is its dependence on changes in the size, shape, or attenuation values of a structure to detect pathologic processes.
Separate CT and PET scans of the same patient acquired on different scanners certainly can be aligned using a number of available software methods, but the algorithms are often labor intensive and, particularly in the abdomen, may fail to provide a satisfactory overlap. However, combined imaging with PET/CT allows for the precise structural information provided by CT to accurately locate the hypermetabolic foci identified with PET and thereby improve the overall diagnostic performance ( Figure 12-1 ). The information from combined PET/CT also facilitates identification of physiologic from pathologic uptake and diminishes the false-negative and false-positive interpretations of the individual components. Indeed, it has been shown that PET/CT is more accurate for many oncologic entities than either CT or PET alone. Furthermore, PET has the potential to be used with radiotracers different from FDG to provide other useful biological information, whereas CT provides useful information about the behavior of orally and intravenously administered contrast agents.

Instrumentation Considerations
The first PET/CT prototype scanner was revealed in 1998. Subsequently, the first clinical scanners appeared in 2001, and by early 2007 more than 800 combined PET/CT scanners had been installed in clinical institutions worldwide. Essentially all new PET machine sales are now as PET/CT scanners.
The automatic and more accurate coregistration of the structural and metabolic data is just one of the inherent advantages of hybrid PET/CT machines. PET-only whole-body scans traditionally required up to 45 to 60 minutes to complete. The transmission scan required for attenuation correction to improve the qualitative and quantitative accuracy of PET is a major contributor to this long scan time. However, CT-based attenuation correction is significantly quicker than traditional PET transmission methods and can provide almost noise-free information and allows for total PET/CT scanning durations of 20 to 30 minutes or less. This greatly reduced scanning time enhances patient comfort and convenience and also allows a greater patient throughput. The current emission PET scan time may now also be decreased by the use of time-of-flight (TOF) technology, which shortens scan time and improves signal-to-noise ratio.
Feasible TOF now can be applied owing to the newer fast scintillators with high stopping power, such as lutetium oxyorthosilicate (LSO) and LYSO (which is LSO with the addition of a small percentage of yttrium). TOF makes use of the ability of the new scanners to measure the arrival time difference between the two positron annihilation-generated photons reaching the detectors to within a certain resolution. Recent detector and scintillator developments allow sub-nanosecond coincidence timing resolution, which provides a rapid, TOF-based and back-projection–free, three-dimensional (3D) reconstruction algorithm that, combined with real-time data acquisition and a quick detector encoding scheme, permits high-quality images to be obtained in a significantly reduced time, particularly in nonobese patients. The stability of these new scanners in practice has yet to be confirmed; and although encouraging and interesting, the precise clinical contribution of TOF PET has yet to be determined. Certainly, high spatial resolution PET detectors appear to optimize the conspicuity of FDG uptake in small lesions.
From the late 1980s until recently, the bismuth germanate (BGO) block detector was the standard for PET. However, the introduction of new faster scintillators with greater light output and shorter decay time than BGO, such as gadolinium oxyorthosilicate (GSO) and LSO (and LYSO), has recently improved the performance of PET scanners for clinical imaging. The faster scintillators decrease the coincidence timing window and thus the random coincidence rate. The improved light output of the new scintillators makes the energy resolution more accurate by reducing the statistical uncertainty of the measurement. This higher light output also improves the positioning accuracy of a block detector, with potential spatial resolution benefits.
The block design developed by Casey and Nutt has been the basic detector component in all multi-ring PET scanners for the past 2 decades. The first multi-ring PET scanners incorporated septa between the detector rings to shield the detectors from scattered photons out of the transverse plane, thus not allowing 3D reconstruction algorithms. The first multi-ring PET scanners with retractable septa that included the capability to acquire data in either the 2D or 3D mode appeared in the early 1990s. The advantages of 3D imaging for the brain are widely accepted, but 3D imaging for the rest of the body has posed more problems. Recently, however, statistically based reconstruction algorithms, more accurate scatter correction methods, and faster scintillators have significantly improved the achievable whole-body 3D imaging quality. However, 2D imaging still may be more suitable for large patients. Increased axial coverage makes better use of the emitted radiation for a given volume of scintillator. For most PET/CT scanners, axial PET coverage is approximately 15 cm, ranging up to a recently extended 21.6 cm, with resulting imaging advantages.
Technique Considerations
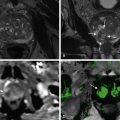
PET/CT continues to be an evolving modality, and thus accepted optimal protocols are not yet established. Debate exists, for example, regarding the use of oral and intravenous contrast material and the optimal respiratory phase to scan as well as the most appropriate CT scanning parameters. FDG-PET provides quantitative information in the form of the standardized uptake value (SUV). SUV estimation requires attenuation correction to avoid the variability in FDG uptake depending on the tumor depth beneath the skin. As mentioned earlier, CT images are used for attenuation correction of the PET emission data, eliminating the need for a separate lengthy PET transmission scan with much less statistical noise. However, there is also a potential risk for overestimating the true FDG activity with this technique. In normal structures, including bone and soft tissues, this is unlikely to be problematic. However, very dense structures on CT such as metallic prostheses, surgical clips, and devices, as well as high-density oral and intravenous contrast can result in overcorrection with subsequent artifact formation. As a consequence, the corresponding photopenic areas on PET may manifest artifactually as hypermetabolic foci. This usually can be recognized by reviewing the nonattenuation corrected PET images. These artifacts, however, also may obscure true foci of abnormal activity on the attenuation-corrected images, although they are most intense at areas of contrast influx, such as the subclavian vein, outside the abdomen. A saline flush after intravenous injection of a contrast agent may reduce attenuation correction PET artifacts. Recent reports suggested that the changes in attenuation correction wrought by intravenous contrast agents are neither statistically or clinically significant. This is reassuring because intravenous contrast agents are regularly used in CT to augment the contrast between normal and abnormal tissues and thus increase the ease of interpretation. They give useful information about the patency ( Figure 12-2 ), course, and relations of vessels ( Figure 12-3 ) and also dynamic data regarding tissue enhancement, perfusion, and de-enhancement.


Modifications to the basic scaling algorithm have been introduced to distinguish oral contrast enhancement from bone, and strategies have been developed that minimize problems caused by both intravenous and oral contrast material. The modified algorithm also can somewhat reduce artifacts from metallic objects in the patient. Because the attenuation values are energy dependent, the correction factors derived from a CT scan at mean photon energy of 70 keV must be scaled to the PET energy of 511 keV. Scaling algorithms typically use a bilinear function to transform the attenuation values above and below a given threshold with different factors. The scaled CT images are then interpolated from CT to PET spatial resolution, followed by reprojection of the interpolated images. Alternatively, fully optimized contrast-enhanced CT can be performed after initial low-mAs PET/CT without intravenous contrast agent administration for attenuation correction, although this may increase radiation exposure to the patient.
Oral contrast agents are also routinely used in CT to assist the CT diagnosis of both gastrointestinal tract–related and neighboring pathologic conditions. In the past, these were generally positive, dense substances including barium and Gastrografin compounds. This again leads to the potential production of erroneous hypermetabolic foci after CT-based attenuation correction. Furthermore, the distribution of such agents may change position owing to peristalsis between the CT used for attenuation correction and the PET components, a further potential confounding phenomenon. Accordingly, some authors support the use of water-attenuation oral contrast agents or even not using any oral agent at all. However, clearly, oral contrast is helpful for CT interpretation and, as with intravenous contrast agents, there is growing consensus that dilute concentrations of even positive oral contrast agents do not significantly alter PET/CT attenuation correction. Optimal PET/CT allows for the ability to delineate the bowel on the CT component and thus differentiate FDG uptake in the colon from that in adjacent tumor sites ( Figure 12-4 ).