The focus of this chapter is on positron emission tomography (PET) and PET and computed tomography (PET/CT) applications specific to the tumors arising in the gastrointestinal tract and female gynecologic organs, excluding systemic malignancies such as lymphoma and melanoma, which can manifest in the abdomen ( Figure 13-1 ), as well as other approved extraabdominal malignancies that can metastasize to the abdomen, such as lung and breast cancers.

Imaging of Specific Areas
Gastrointestinal Tract
Esophageal Carcinoma
Because it is mainly an intrathoracic organ, the esophagus will not be discussed except to note that the incidence of adenocarcinoma of the distal esophagus and gastroesophageal junction has increased dramatically in recent years, currently accounting for the majority of new cases of esophageal cancer. The Centers for Medicare and Medicaid (CMS) guidelines in the United States approve fluorodeoxyglucose (FDG)-PET, and thus PET/CT, for the diagnosis, staging, and restaging of patients with esophageal cancer, including both squamous cell carcinoma and adenocarcinoma subtypes ( Figure 13-2 ).

Colorectal Carcinoma
Colorectal cancer is the malignant abdominal tumor that has been extensively investigated with FDG-PET and PET/CT. The CMS guidelines in the United States have approved Medicare coverage for FDG-PET, and thus PET/CT, for the diagnosis, staging, and restaging of patients with colorectal cancer.
Diagnosis.
FDG-PET is sensitive for detecting primary colorectal carcinoma (95% to 100%), but it has unacceptably lower specificity than conventional modalities at initial staging. In practice, FDG-PET is therefore rarely specifically used for the diagnosis of colorectal cancer, although it may incidentally detect such a tumor as PET, and especially PET/CT, becomes more extensively used. At the primary site, however, the positive predictive value of PET is lower than the negative predictive value, owing to the false-positive FDG-PET findings of both physiologic bowel activity and inflammatory processes.
Staging.
In staging patients with colorectal cancer, FDG-PET and PET/CT are mainly used to assess regional lymph node involvement and distant metastases. However, FDG-PET has been shown to have a very low sensitivity of between 22% and 29% for regional lymph node metastases, with CT also demonstrating low values (29%). However, FDG-PET specificity was superior (96% vs. 85%). False-negative findings in regional metastatic lymph nodes on FDG-PET are considered to be due to low sensitivity for microscopic involvement of small lymph nodes or occasionally as a result of increased FDG uptake by the primary tumor, which masks the immediately neighboring structures.
Most patients with colorectal cancer at initial staging have disease limited to the colon or to regional pericolic or mesenteric lymph nodes. For patients with early colorectal cancer, surgery is usually performed with the intention to achieve cure. However, distant metastatic disease may necessitate the surgery for complications, which include hemorrhage, obstruction, and perforation. Given this role of surgery in both regional and advanced disease, surgical and pathologic criteria define the colorectal cancer staging classification.
The standard tumor, node, metastasis (TNM) classification is currently advocated by the American Joint Committee on Cancer. Because of inherent technologic limitations, imaging modalities today are generally unable to match the diagnostic staging information provided by surgical and pathologic findings. PET cannot provide an accurate T-stage determination necessary for TNM nomenclature, in which exact depth of invasion is the primary parameter. PET is usually accurate only in cases of gross serosal penetration and invasion of neighboring structures. CT gives more exact structural information but, unfortunately, usually also cannot adequately resolve the bowel wall layers. N staging necessitates evaluation of mesenteric nodes as well as pericolic nodes, which are frequently small and lie beside the primary tumor. Furthermore, pericolic nodes again are often involved microscopically by tumor, which usually can be diagnosed only by histopathologic evaluation. Despite the clear superiority of surgery and histopathology at TNM staging, preoperative imaging detection of nodal or organ metastases is important in guiding the general management toward palliation or curative tumor resection. Notwithstanding its staging limitations, PET/CT offers combined metabolic and structural information that is particularly useful in cases of more advanced local disease.
Metastatic Disease.
Disease spread beyond the regional pericolic or mesenteric lymph nodes represents metastatic disease. Lymphatic spread usually involves internal iliac or retroperitoneal nodes, and hematogenous spread of colorectal cancer usually involves the lungs or liver ( Figure 13-3 ). Metastases to other sites in the absence of pulmonary or hepatic involvement are relatively unusual. A major advantage of PET as well as PET/CT is the accurate detection of distant metastases.

Liver metastases, if recognized early enough, may be treated by neoadjuvant chemotherapy and resection, which may lengthen survival for patients with colorectal cancer. Both PET and CT are accurate for diagnosis of metastases to the liver. Multidetector row CT following intravenous administration of a contrast agent is a standard primary imaging modality for the assessment of focal liver lesions, with uncertain liver lesions generally evaluated with enhanced MRI. In a meta-analysis performed by Kinkel and colleagues, PET was considered superior to older CT technology for the detection of liver metastases but appears limited in its ability to demonstrate subcentimeter lesions. Sahani and associates showed that gadolinium-enhanced MRI outperformed PET in assessing liver metastases from colorectal and pancreatic cancer, again particularly for small lesions. In addition, PET alone does not provide adequate anatomic information for satisfactory surgical planning regarding the precise localization of metastases according to hepatic segments or with respect to the positioning of lesions in relationship to or involvement of vessels or gallbladder. There are, in general, very few studies comparing PET or PET/CT with modern CT or MRI technique, but a relatively recent study by Chua and coworkers compared PET/CT with CT with intravenous contrast for the evaluation of patients with hepatic metastases. In patients with colorectal carcinoma, PET/CT had 94% sensitivity and 75% specificity compared with inferior values of 91% and 25%, respectively, for CT. Furthermore, PET/CT may be particularly helpful in patients with fatty liver and hypodense or hypoenhancing liver lesions that are not clearly characterized by CT alone and in patients with an increasing carcinoembryonic antigen (CEA) value in whom CT fails to detect metastases.
The most important role of PET in patients with hepatic metastases is in the diagnosis of extrahepatic metastatic foci that would preclude a curative tumor resection. Several investigators have examined the incremental value of PET as a supplement to CT and found that PET offers significant management information beyond that from CT alone. Investigators have found that when PET is added to CT in preoperative planning for patients with hepatic metastases, additional extrahepatic metastatic foci are identified in 11% to 23% of patients and can result in prolonged patient survival after institution of appropriate patient management. This is often due to a treatment change to a systemic approach with chemotherapy rather than localized therapy.
In patients with elevated CEA levels, FDG-PET can identify metastases previously unrecognized on conventional diagnostic modalities. Valk and coworkers found that an average of $3003 was saved per patient when PET was added to the preoperative diagnostic workup; FDG scanning was able to differentiate patients with unresectable disease rather than resectable disease, thus avoiding futile operations. This reduction in unnecessary procedures was supported by a study by Park and colleagues. Management was altered in 27 patients based on PET/CT results; 9 had intermodality changes, 10 received more extensive surgery, and 8 avoided futile procedures. PET/CT improved the management plan in 24% of patients with primary colorectal cancer.
Restaging.
As during the initial staging of colorectal cancer, FDG-PET has been reported to be more accurate overall than CT for the presence of metastatic disease. An early study by Hung and colleagues compared FDG-PET, CT, and serum CEA for the evaluation of recurrent colorectal disease. They reported sensitivity and specificity of FDG-PET to be 100% and 83%, respectively, compared with 33% and 86%, respectively, for CEA. Abdominal CT had a lower sensitivity and specificity of 78% and 61% for detecting local recurrence and detected one lymphatic and one hepatic metastasis. They concluded that FDG-PET was more accurate than CT and CEA for the detection of recurrent colorectal cancer. In a recurrent colorectal carcinoma meta-analysis study by Wiering and colleagues, pooled sensitivity and specificity were 88% and 96%, respectively, for FDG-PET in the detection of hepatic metastases compared with lower values of 83% and 84%, respectively, for CT. For extrahepatic disease, pooled sensitivity and specificity for PET were 92% and 95%, respectively, in comparison to 61% and 91% for CT. Clinical management changed in 32% based on the PET findings.
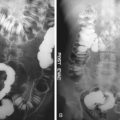
The imaging distinction of postoperative inflammation, scarring, radiation fibrosis, and other sequelae of prior therapy from recurrence is challenging in patients with prior colorectal carcinoma. This is most problematic with rectal tumors, in which presacral scarring and pelvic desmoplastic changes are common findings. With conventional imaging, serial examinations are frequently required before slowly developing malignant changes can be appreciated. When PET is performed more than 6 months after surgery, a time when postsurgical change is not hypermetabolic unless there is a leak causing persistent inflammation, increased FDG uptake in the presacral space generally indicates tumor recurrence. PET/CT at this time has been shown to be highly accurate for differentiation of benign from malignant presacral changes, with reported sensitivity, specificity, and positive and negative predictive values of 100%, 96%, 88%, and 100%, respectively ( Figure 13-4 ). The first published study of PET/CT of colorectal cancer reported that the staging and restaging accuracy improved from 78% with PET alone to 89% with PET/CT. The frequency of equivocal and probable lesion characterization was halved, and the superiority of PET/CT over CT or PET alone has become more established with numerous studies demonstrating improved results. Furthermore, PET/CT as a single study is more accurate for recurrent colorectal cancer than coregistration of two separately acquired PET and CT studies.

Future Positron Emission Tomography and Computed Tomography Applications for the Patient With Colorectal Disease.
PET/CT will continue to play an important role in determining the resectability status of patients with recurrent rectal carcinoma before surgery and in the planning of aggressive procedures ( Figure 13-5 ) but is limited in defining local invasion of rectal tumors to adjacent tissues. Assessment of neoadjuvant therapy and guidance for radiation therapy are other applications that may be particularly suited to PET/CT. CT colonography (virtual colonoscopy) is a recently approved approach for the evaluation of patients at risk for colon cancer. It is possible combined PET/CT colonography will add specificity by selectively identifying hypermetabolic polyps, likely carrying a higher risk for malignant degeneration.

Other Gastrointestinal Malignancies
PET and PET/CT have been used to assess patients with a number of other malignancies associated with the gastrointestinal tract and other abdominal organs. Although many of these are not currently covered by the CMS in the United States, the National Oncologic PET Registry (NOPR) has allowed their use for such diseases if patients are suitably enrolled, and with time many of these malignancies may gain full coverage. Some of these therefore will be briefly discussed here.
Gastric Cancer.
Early-stage gastric carcinoma has a relatively good prognosis. However, unfortunately patients usually present late with advanced disease and, as a result, it is the second leading cause of cancer death worldwide. Where screening for gastric cancer occurs, the prognosis is better because the malignancy is detected at an earlier stage.
The role of FDG-PET and PET/CT in gastric cancer remains controversial. Despite a positive initial study by Yeung and colleagues, most subsequent studies have demonstrated poorer rates of primary lesion detection, including a study by Shoda and coworkers, which reported just 10% sensitivity and 99% specificity for early gastric cancer. This has led some authors to conclude that FDG-PET is not a suitable first-line diagnostic procedure in the detection of gastric cancer and is not helpful in tumor staging. There are a number of reasons for the relatively poor performance of FDG-PET in gastric cancer. First, normal physiologic FDG uptake in the stomach and gastroesophageal junction is variable and can have a focal appearance, especially if the stomach is empty and collapsed or after partial gastrectomy for malignancy. Benign inflammatory conditions demonstrate increased uptake, which can result in false-positive results. It has been proposed that the sensitivity of PET/CT may be improved upon with the ingestion of water or neutral density contrast to distend the stomach at the time of scanning. Stahl and associates reported variable FDG uptake to be dependent on differing histopathologic findings, which may help explain the variable results for FDG-PET in gastric carcinoma. They reported overall 60% sensitivity for the detection of locally advanced gastric cancers. Within this group, the detection rate was higher for intestinal type at 83% versus just 41% for the diffuse type. The mean standard uptake value was also significantly lower for mucus-containing versus non–mucus-containing tumors ( Figure 13-6 ).