Pediatric brain tumors are the leading cause of death from solid tumors in childhood. The most common posterior fossa tumors in children are medulloblastoma, atypical teratoid/rhabdoid tumor, cerebellar pilocytic astrocytoma, ependymoma, and brainstem glioma. Location, and imaging findings on computed tomography (CT) and conventional MR (cMR) imaging may provide important clues to the most likely diagnosis. Moreover, information obtained from advanced MR imaging techniques increase diagnostic confidence and help distinguish between different histologic tumor types. Here we discuss the most common posterior fossa tumors in children, including typical imaging findings on CT, cMR imaging, and advanced MR imaging studies.
Key points
- •
Medulloblastoma is the most common posterior fossa tumor in children.
- •
Due to high cell density and high nuclear-to-cytoplasmic ratio, medulloblastomas are typically hyperdense on computed tomography, isointense to the cerebellar cortex on T2 and present with restricted diffusion, as well as a very high choline peak and a taurine peak on magnetic resonance spectroscopy.
- •
Cerebellar pilocytic astrocytoma is a World Health Organization grade I tumor with a solid portion that is typically hyperintense to the cerebellar cortex on T2 due to high water content along with low cell density.
- •
Extension through the fourth ventricular outflow foramina, although typical, is not entirely pathognomonic of ependymoma.
- •
Brainstem gliomas are usually located in the pons, with diffuse midline glioma H3 K27-mutant the most common.
Introduction
Pediatric brain tumors are the leading cause of death from solid tumors in childhood. The most common posterior fossa tumors in children are medulloblastoma (MB), atypical teratoid/rhabdoid tumor (ATRT), cerebellar pilocytic astrocytoma (CPA), ependymoma, and brainstem glioma (BG). Location, as well as imaging findings on computed tomography (CT) and conventional magnetic resonance (cMR) imaging may provide important clues to the most likely diagnosis. Moreover, information obtained from advanced MR imaging techniques, such as diffusion-weighted imaging (DWI), MR spectroscopy (MRS), perfusion-weighted imaging, and dynamic contrast-enhanced (DCE) studies, increase diagnostic confidence and help distinguish between different histologic tumor types.
Here we discuss the most common posterior fossa tumors in children, including typical imaging findings on CT, cMR imaging, and advanced MR imaging studies.
Introduction
Pediatric brain tumors are the leading cause of death from solid tumors in childhood. The most common posterior fossa tumors in children are medulloblastoma (MB), atypical teratoid/rhabdoid tumor (ATRT), cerebellar pilocytic astrocytoma (CPA), ependymoma, and brainstem glioma (BG). Location, as well as imaging findings on computed tomography (CT) and conventional magnetic resonance (cMR) imaging may provide important clues to the most likely diagnosis. Moreover, information obtained from advanced MR imaging techniques, such as diffusion-weighted imaging (DWI), MR spectroscopy (MRS), perfusion-weighted imaging, and dynamic contrast-enhanced (DCE) studies, increase diagnostic confidence and help distinguish between different histologic tumor types.
Here we discuss the most common posterior fossa tumors in children, including typical imaging findings on CT, cMR imaging, and advanced MR imaging studies.
Medulloblastoma
Medulloblastoma (MB), a highly malignant neoplasm, is the most common posterior fossa neoplasm in children, representing 15% to 20% of all pediatric brain tumors and 30% to 40% of posterior fossa neoplasms. Medulloblastomas are classified as embryonal tumors, the largest group of malignant tumors in the pediatric population.
This highly malignant neoplasm occurs more frequently in boys, usually before 10 years of age. Although less common, the disease may also occur in adults, usually in the third and fourth decades of life.
Clinical Picture and Treatment
Clinical symptoms and signs are generally brief, typically less than 3 months in duration, and reflect the strong predilection of this tumor to arise within the cerebellum, most often in the vermis. Symptoms may include headache, general malaise, failure to thrive, vomiting, and clumsiness, among other presentations that mimic common and benign childhood pathologies seen in primary care.
Typically, the treatment strategies for MB are threefold: maximal safe resection (which may include cerebrospinal fluid [CSF] diversion), neuraxis radiotherapy, and chemotherapy.
Location
The tumor usually arises at the midline within the vermis and exhibits growth into the fourth ventricle ( Fig. 1 ).
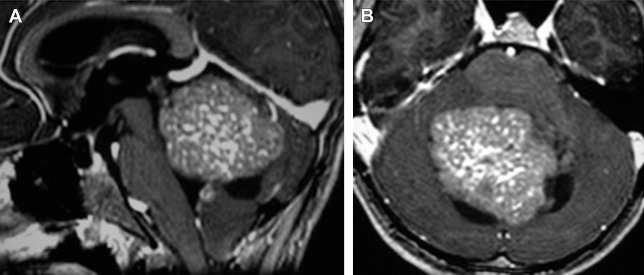
Less typical locations include nonventricular superior or inferior vermian tumor, cerebellar hemispheric lesions, and extension into the foramina of Magendie and foramina of Luschka to the cerebellopontine angle (CPA).
Computed Tomography and Conventional MR Imaging
On unenhanced CT, the tumor is usually characterized as hyperdense ( Fig. 2 ), and on T2 images, as isointense to hypointense compared with gray matter ( Fig. 3 ). These imaging findings are likely secondary to high cell density and high nuclear-to-cytoplasmic ratio.
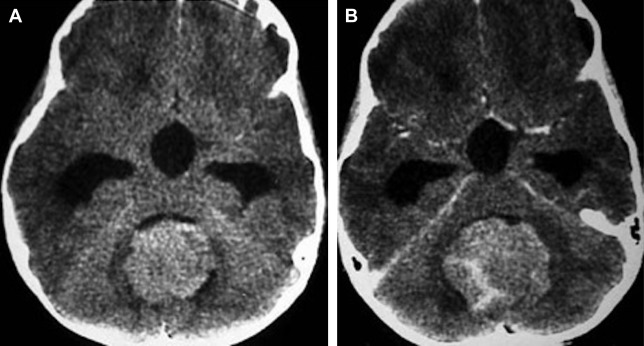
The tumor typically may appear heterogeneous on imaging, with findings related to cyst formation and hemorrhage on MR, and calcification seen on CT (see Fig. 3 A–C). Intratumoral cyst or necrosis is observed in 40% to 50% of cases.
MBs typically enhance.
Atypical imaging findings, such as high signal intensity compared with the cerebellar cortex on T2, as well as no enhancement may be demonstrated ( Fig. 4 ).
Sometimes the tumor presents with an infiltrative pattern instead of a solid solitary mass ( Fig. 5 ).
Evidence of leptomeningeal metastatic spread is present in 33% of all cases at the time of diagnosis and is well evaluated with contrast-enhanced MR imaging of the brain and the spine. MR imaging is more sensitive than CSF studies for the detection of CSF spread of primary brain tumors.
Metastases may be leptomeningeal, dural-based, intraventricular, adherent to the spinal roots, or even to the liver ( Fig. 6 ).
Metastasis to the brain parenchyma may bleed, resembling cavernoma ( Fig. 7 ).
Diffusion-Weighted MR Imaging
Apparent diffusion coefficient (ADC) values are significantly lower in MBs than in all other posterior fossa tumors ( P <.001) related to high cell density ( Fig. 8 , see also Figs. 3 D, E and 5 E).
A study by Jaremko and colleagues confirmed that diffusion imaging is the single most useful sequence for differentiating pediatric posterior fossa tumors and that, as expected, diffusion restriction is rare in grade 1 tumors and common in grade 4 tumors. The optimal threshold for distinguishing MB and juvenile pilocytic astrocytomas (JPAs), ADC minimum = 800 × 10 −6 mm 2 /s, was lower than the threshold of 900 × 10 −6 mm 2 /s used by Rumboldt and colleagues, likely because they used ADC mean rather than ADC minimum.
Desmoplastic medulloblastoma, a histologically less aggressive subtype with better prognosis than the classic type, is expected to have less highly restricted diffusion than the classic type. Some of these tumors present with no restricted diffusion at all ( Fig. 9 ).
Proton Magnetic Resonance Spectroscopy
Choline
On MRS, MBs usually demonstrate a significant elevation of the choline (Cho) peak related to high cell density and elevated Cho/Cr and Cho/N-acetyl-aspartate (NAA) ratios, reflecting its malignant nature ( Fig. 10 , see also Figs. 3 F and 5 F).
High Cho has been previously reported as a characteristic finding of embryonal tumors.
Elevation of the Cho peak is useful in distinguishing between MB and L’Hermitte-Duclos disease (LDD), as MBs occasionally may present with a laminated appearance, and with no contrast enhancement, mimicking LDD. The Cho peak is typically elevated in patients with MB when compared with patients with LDD.
Desmoplastic MBs may present with no elevation of the choline in the spectra. In these tumors, a huge myo-inositol peak may be seen related to the desmoplastic nature (Lara A. Brandão, MD, personal communication, 2013) ( Fig. 11 ).
Taurine
Spectra with a short echo time (TE) show a significantly elevated taurine (Tau) concentration at 3.3 ppm in patients with MB when compared with other tumors (see Fig. 10 ). Furthermore, at a TE of 30 ms, the Tau peak projects above the baseline; whereas, at a TE of 144 ms, the Tau peak occurs below the baseline.
Tau has been established as an important biomarker in distinguishing MBs from other common pediatric brain tumors, such as cerebellar astrocytomas. Higher Tau levels are associated with increased cellular proliferation and tumoral aggressiveness.
Glutamine and glutamate and alanine
In a study of 60 children with untreated brain tumors, Panigrahy and colleagues measured the highest glutamate (Glu) concentrations in pineal germinoma and in MB (see Fig. 10 B). Specifically, the MB, pineal germinoma, and astrocytoma showed mean glutamine and glutamate (Glx) concentrations above the mean in all tumors; whereas, Glx concentration was low in both the choroid plexus papilloma and carcinoma. The quantitation of these metabolites proved useful in separating either MB or astrocytoma from choroid plexus papilloma. Panigrahy and colleagues have also reported the highest mean alanine (Ala) concentration among posterior fossa tumors in MBs.
Lipids and lactate
Prominent lipid (Lip) resonances can be observed in some, but not all, spectra of malignant MB (see Fig. 11 B).
High lactate (Lac) values are usually found in the spectra of MB.
Magnetic resonance spectroscopy in metastatic versus localized medulloblastomas
Metastatic MBs are characterized by higher total Cho (tCho), which is consistent with increased cell turnover and tumor growth, a finding substantiated by a significant positive correlation between tCho and the Ki67 index. Tau is present in both metastatic and localized tumors, although higher levels are typically found in metastatic tumors, which is consistent with previous findings in neuroblastoma (ie, Tau is a reliable biomarker for more aggressive subtypes of neural tumors). The fact that higher mobile Lip levels are observed in localized tumors may also reflect a higher proportion of necrotic tumor in these cases.
Dynamic Susceptibility Contrast and Dynamic Contrast-Enhanced MR Imaging
There can be variable perfusion and permeability characteristics in MB, with some lesions showing elevated perfusion and permeability and others not ( Fig. 12 , see also Fig. 3 H).
Important considerations
Radiologic-pathologic correlation
The World Health Organization (WHO) classification system 2007 uses histology to classify MBs into 4 major groups, including classic, desmoplastic, MB with extensive nodularity (MBEN), and large cell/anaplastic MB subtypes :
Classic
Classic MB represents the most common histologic subtype and is composed of sheets of densely packed small round blue cells (basophilic) with a high nuclear-to-cytoplasmic ratio, mitotic and apoptotic activity, and may occur in the midline.
Elevation of Tau is seen specifically in this histologic subtype.
Desmoplastic
This subtype is hypocellular, presents with lower Tau concentration compared with the classic subtype and carries a favorable prognosis.
This histologic subtype is often found in adult patients with MB, demonstrating a cerebellar hemispheric mass extending to the overlying meninges, with desmoplastic reaction evoked by prominent leptomeningeal involvement ( Fig. 13 ).
Anaplastic
Anaplastic MBs (15%) are characterized by marked nuclear pleomorphism, nuclear molding, and cell–cell wrapping, and the large cell variant (2%–4%) displays a monomorphous population of large cells whose nuclei exhibit prominent nucleoli. Both variants are characterized by a very high proliferative activity, abundant apoptosis, and a much poorer prognosis.
This is the most aggressive subtype, characterized by presence of necrosis.
Extensively nodular
MBENs tend to develop in the vermis in children younger than 3 years in most cases and is frequently represented as a nodular enhancing appearance on CT scans or MR images. Prognosis is better than for the classic MB.
Molecular subgroups
More recently there has been the development of a classification of 4 main subgroups of MBs based on molecular profiling.
The WNT and SHH groups were named after the predominant signaling pathways thought to be affected in their pathogenesis. Less is known currently regarding the pathogenesis of groups 3 (tending to harbor MYC amplification) and 4 (tending to have isochromosome 17q) and therefore generic names were chosen until they are better understood.
The SHH group has become of increasing interest because of the availability and temporary success of small molecule inhibitors to smoothened (SMO), which is part of the SHH pathway.
For a detailed comprehensive review on the molecular subgroups of MB, see the consensus article by Taylor and colleagues.
MB is the most common malignant brain tumor in children and, as such, has been the focus of tremendous efforts to genomically characterize it.
What was once thought to be a single disease has been divided into multiple, molecularly unique subgroups through gene expression profiling. Each subgroup is not only unique in its origin and pathogenesis, but also in the prognosis and potential therapeutic options. The molecular classification system has a potential use in developing prognostic models as well as for the advancement of targeted therapeutic interventions.
MB is currently stratified into 4 molecular variants through the advances in transcriptional profiling.
They include sonic hedgehog (SHH), wingless (WNT), Group III, and Group IV.
SHH (sonic hedgehog) medulloblastomas
SHH tumors are thought to account for 28% of all medulloblastomas.
They have an intermediate prognosis between good prognosis WNT tumors and poor prognosis group 3 tumors, and may be similar in prognosis to group 4. SHH MBs show a dichotomous age distribution being more common in both infants (<4 years) and adults (>16 years).
Most tumors in this group are of the desmoplastic subtype, located in the cerebellar hemisphere more often than in the midline.
WNT (wingless) medulloblastomas (∼10%)
WNT tumors are thought to be the rarest subgroup of medulloblastoma, accounting for 11% of these tumors, but they have probably been the most studied and have a very good long-term prognosis with overall survivals reaching 90%.
WNT tumors also show a specific age distribution being almost absent in infants (aged <4 years) but predominantly affecting children with a peak incidence of 10 to 12 years.
Most (97%) WNT MBs show classic histology; however, rarely, they are phenotypically large cell/anaplastic and may remarkably retain their relatively good prognosis with this phenotype. They tend to occur in the middle cerebellar peduncle/cerebellopontine angle.
Group 3
Group 3 tumors account for 28% of all MBs.
They are associated with the worst prognosis of all the subgroups and are frequently metastatic. Group 3 tumors are found in infants and children but very rarely in adults. Group 3 MBs are mostly classic or large cell/anaplastic morphology. MYC amplification appears to be highly associated with group 3 tumors and is associated with a worse prognosis. The tumors in this subgroup tend to be ill-defined on imaging.
Group 4
Group 4 MBs are thought to be the most common “typical” subgroup of MB, accounting for approximately 34%, and can be thought of conceptually as being associated with isochromosome 17q. Group 4 medulloblastomas rarely affect infants (0–3 years) and mainly affect children, with a peak age of 10 years.
Although they frequently metastasize, they still have an intermediate prognosis compared with the poor prognosis of group 3.
The vast majority of group 4 MBs have a classic histology.
All histologic subtypes can present with this molecular profile, except the desmoplastic one.
These tumors tend to have minimal or no enhancement.
Molecular profiling: implications in treatment
The identification of different molecular pathways involved in the pathogenesis of MBs provides new therapeutic targets for drug development.
Medulloblastomas and associated syndromes
Basal cell nevus syndrome (Gorlin syndrome)
This is a rare autosomal dominant disorder with high incidence of neoplasms, notably MB. Ten percent of these patients will develop MBs, usually desmoplastic.
Falcine calcification in children with MB may be a marker for basal cell nevus syndrome.
Turcot syndrome
Turcot syndrome is associated with familial colonic polyposis, with high incidence of brain tumors, such as MB and glioma.
Li-Fraumeni
Germline mutations of the p-53 tumor suppressor gene predisposes to different types of cancer in patients, especially soft tissue sarcomas. Ten percent of these patients develop MB.
Key points to remember
- •
MB is the most common posterior fossa tumor in children
- •
MB affects mainly boys before 10 years of age
- •
There is a second peak in adults
- •
Lesion is often located in the midline vermis and presents with hyperattenuation on CT, isointense to hypointense on T2, restricted diffusion and high Cho and taurine on MRS
- •
Perfusion and permeability values are variable
- •
Look for CSF spread!
- •
There are imaging features associated with molecular subgroups: SHH involves the cerebellar hemisphere, the WNT pathway involves cerebellar peduncle/CPA cistern, group 3 tumors are ill-defined on imaging, and group 4 tumors have minimal or no enhancement
Atypical teratoid rhabdoid tumors
ATRTs are classified as part of the embryonal tumor group of central nervous system (CNS) tumors.
ATRT is a highly malignant CNS neoplasm that most often occurs in children younger than 2 years. ATRTs represent 1.3% of CNS primary brain tumors in the pediatric population, but if one considers only children younger than 3, prevalence rises to 20%.
ATRTs are more common in girls than in boys, with 94% in an intra-axial location. A review of 14 histologically confirmed cases of ATRTs demonstrated equal preference for the supratentorial and infratentorial compartments.
These tumors are aggressive lesions with a dismal prognosis, and a 2-year survival of only 17%. Survival improves if the patient is older than 3 years.
Poor prognosis is related to the young age of the affected patients as well as the high propensity for CSF tumor spread. Metastasis to the lungs and abdomen also may be demonstrated.
Imaging Findings
On unenhanced CT, the tumor is usually characterized as hyperdense ( Fig. 14 ) and on T2 images, as isointense to hypointense compared with gray matter ( Fig. 15 ). These imaging findings are likely secondary to high cell density and high nuclear-to-cytoplasmic ratio and overlap with those described for MBs.
Enhancement is demonstrated in approximately 89% of the cases (see Fig. 15 F).
Due to high cell density, as well as high nuclear-to-cytoplasmic ratio, restricted diffusion is typically seen (see Fig. 15 G, H).
MRS shows elevated Cho and reduced NAA as well as a prominent Lip peak. However, there are no reports in the literature that quantify these changes or address the presence or size of Tau peaks.
Atypical Teratoid Rhabdoid Tumors Versus Medulloblastoma
The main differential diagnosis for posterior fossa ATRT is MB. If the patient is younger than 3 years, if tumor is located off midline, extending to the CPA, and if blood products are demonstrated in the lesion, one should consider ATRT as the most likely diagnosis.
However, the precise distinction can be made only through immunohistochemistry and genetic analyses. ATRTs frequently demonstrate deletions of chromosome 22q with inactivation of the INI1/hSNF5, thought to be a tumor suppressor gene. Loss of the INI1 gene product is used to diagnose ATRT, although it is not present in all ATRT tumors.
Cerebellar pilocytic astrocytoma
CPA and MB each constitute approximately 35% of all posterior fossa masses in children.
Pilocytic astrocytomas (PAs) are low-grade (grade I) tumors, most often located in the posterior fossa (60%), with 40% involving the cerebellum and 20% involving the brainstem.
CPA has excellent survival after gross total surgical resection.
Differential diagnosis between PA and MB in the posterior fossa is crucial; the former is a low-grade (WHO grade I) tumor, with excellent prognosis, whereas MB is a grade IV tumor, with poorer prognosis.
Location
Predilection for the cerebellar hemisphere instead of the cerebellar vermis is typically demonstrated. The lesion displaces and compresses the fourth ventricle ( Fig. 16 A, B ), as opposed to what is typically demonstrated in MB that usually compromises the cerebellar vermis, filling the fourth ventricle ( Fig. 16 C, D).

Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree





