Early Postoperative Liver Transplant Ultrasound
Introduction
Patients who have undergone liver transplantation are susceptible to a wide variety of complications that can threaten both allograft and patient survival. Some complications are of the kind seen in any patient who has undergone a major surgical procedure or is receiving immunosuppressive drug therapy, but other complications are unique to the surgical transplantation procedure performed.1
The main postoperative complications may be grouped into:
- Vascular abnormalities
- Nonvascular abnormalities
- — Graft rejection
- — Biliary tract complications
- — Localized or systemic infection
- — Graft rejection
- Miscellaneous complications
The clinical diagnosis and management of these complications may be difficult as the clinical and laboratory findings are nonspecific,1 with imaging, especially ultrasonography, providing the necessary stimulus to further diagnostic or interventional procedures.
Ultrasound is normally the first line of evaluation in the post-transplant phase; used either as an investigative tool, when there is clinical evidence of graft dysfunction, or routinely for the early detection of complications before there is any clinical suspicion of abnormality. A detailed ultrasound examination evaluating the biliary system, hepatic vasculature, hepatic parenchyma, and perihepatic spaces may add information crucial to the patient management. Prior to performing an ultrasound examination it is imperative to know the type of surgical procedure that has been performed, as numerous variations exist from transplant of the whole liver to transplant of a single liver lobe or segmental liver transplantation. In orthotopic liver transplantation (OLT), revascularization of the allograft requires anastomoses of the hepatic artery, portal vein, and inferior vena cava (IVC), with biliary tract reconstruction performed to establish bile drainage (Fig. 10.1). Many different anastomoses may be performed according to the preference of the surgeon, the anatomy of the donor and recipient vessels, as well as the underlying disease.
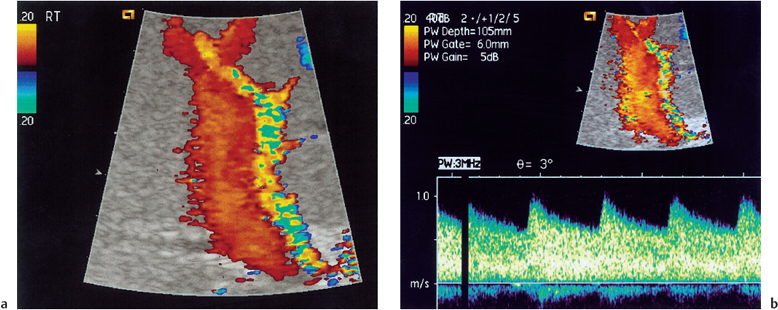
Fig. 10.1 a Normal color Doppler imaging of the portal vein/hepatic artery. b Normal spectral Doppler appearances of the post-transplant hepatic artery
Vascular Complications
Hepatic Artery
In the first two weeks following liver transplantation, routine color Doppler ultrasonography (CDUS) plays an important role in the detection of both clinically evident and clinically unsuspected vascular complications, particularly hepatic artery thrombosis. There is evidence to suggest that routine CDUS of all the hepatic vessels on the first day following liver transplantation and subsequently every three days in the early postoperative phase is beneficial.2 Our practice is to perform CDUS in the first 24 hours after liver transplantation, then to perform a repeat ultrasound at day 4 and finally another prior to discharge. Any clinical change would prompt immediate CDUS of the hepatic artery, as after transplantation the donor biliary system is entirely dependent on hepatic arterial blood supply, in particular the right hepatic artery.3
Hepatic Artery Thrombosis
The quoted incidence of hepatic artery thrombosis (HAT) is 5% of adult and 9-18% of pediatric transplant patients.4 HAT (Fig. 10.2) is a serious complication with a mortality of 50-58 %; retransplantation is often required, and even after retransplantation mortality remains high at 27-30%.5
The ultrasound features of HAT include an absence of color and spectral Doppler flow, with a wall “thump” on Doppler imaging. Ultrasound microbubble contrast medium, by markedly increasing the intensity of the Doppler signal, may help to prevent false-positive diagnosis of HAT.6 HAT is most common in the first six weeks following transplantation,7 with CDUS interrogation of the vessels recommended in the first 24 hours after liver transplantation; the highest incidence of vascular complications in patients without symptoms occurs in this period.2 Collateral formation (Fig. 10.3) very rarely occurs in adults, and takes at least three weeks to become apparent, but it is more common in children.
Clinically, impending or complete occlusion of the hepatic artery is often indicated by generalized nonspecific deterioration of the liver function tests, with a characteristic rise in the AST, or with indications of leakage of bile.8 Tzakis et al.9 described three clinical presentations of HAT that occur with equal frequency:
- — Massive hepatic necrosis, a dramatic clinical emergency, requiring retransplantation for survival
- — Delayed biliary leak as a consequence of bile duct ischemia and necrosis
- — Intermittent episodes of sepsis without an evident source
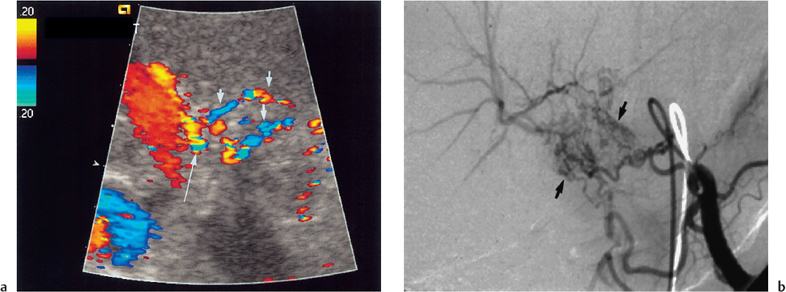
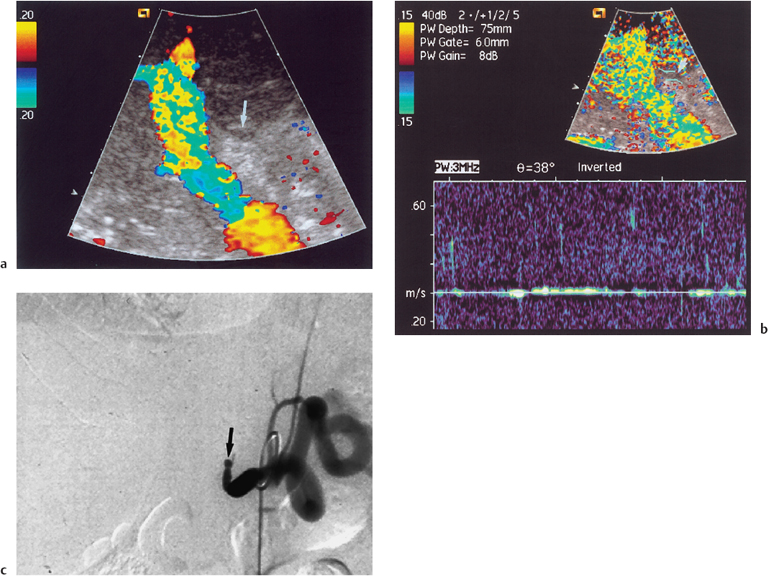
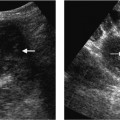
Fig. 10.3 a Color Doppler image of the portal vein and intrahepatic hepatic artery demonstrates collaterals (arrows) forming an intrahepatic hepatic artery branch (long arrow) following long-standing hepatic artery occlusion. b Corresponding selective celiac axis angiogram demonstrating collateral formation (arrows) around an occluded hepatic artery. (Reproduced from Clinical Radiology16 by permission of the Editor.)
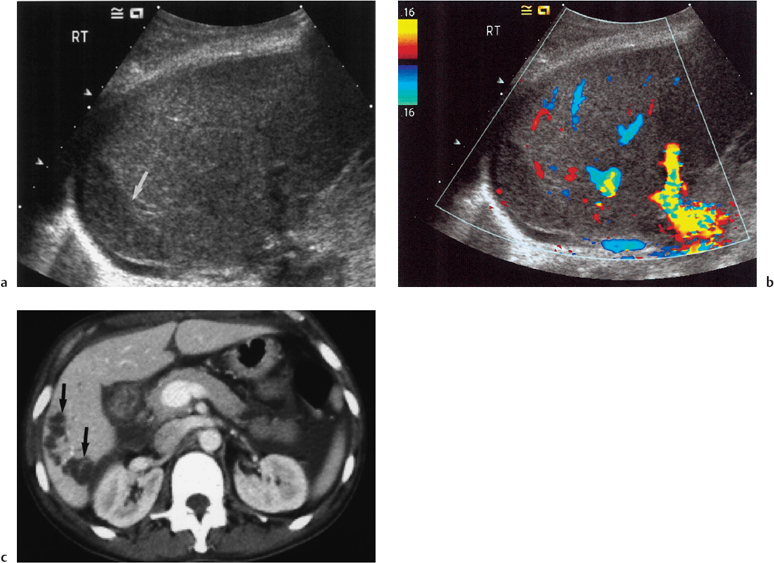
Fig. 10.4 a B-mode ultrasound examination of a transplant liver demonstrating an area of low reflectivity corresponding to an area of infarction (arrow). b Color Doppler image of same region showing no color signal in the infarcted area. c Contrast enhanced CT in a different patient demonstrating focal low-attenuation areas of infarction in a transplant liver (arrows). (Courtesy of Dr. P.A. Kane)
B-mode ultrasonography will demonstrate areas of ischemia as areas of inhomogeneity and decreased echogenicity (Fig. 10.4), but this is a late development and revascularization will not save the graft.
In the early postoperative period, the spectral Doppler waveform can be quite variable, from the high forward diastolic flow to absence of flow in diastole (Fig. 10.5). Absence or reversal of flow in diastole does not appear to indicate a predisposition to thrombosis in the immediate postoperative period.7 While absence of any color or spectral Doppler flow in the hepatic artery is suggestive of thrombosis in the immediate post-transplant period, other factors may contribute to an undetectable hepatic artery signal.5 An increased RI occurs, as the newly transplanted liver is relatively ischemic, with reperfusion edema persisting for up to 72 hours. Furthermore, any cause of edema or inflammation in the transplant may produce a similar result; viral hepatitis or rejection can cause severe edema that can markedly damp the hepatic artery waveform.5 One early study suggested that CDUS has a sensitivity of 92% in the diagnosis of HAT,10 but a more recent study suggests a lower value of 82%, and a positive predictive value of 64-68%.5 Our experience suggests that CDUS of the hepatic artery will visualize 96 % of hepatic arteries in the post-transplant period, and further interrogation of the nonvisualized hepatic artery using ultrasound microbubble contrast medium improves de-
tection to 98.7% (Fig. 10.6).6 Using microbubble contrast has the advantage of reinforcing the signal from the hepatic artery in patients with diminished but present hepatic arterial flow, and precludes the need for more invasive imaging with angiography. Patients who still demonstrate absence of flow following contrast-enhanced CDUS should undergo selective hepatic artery angiography.
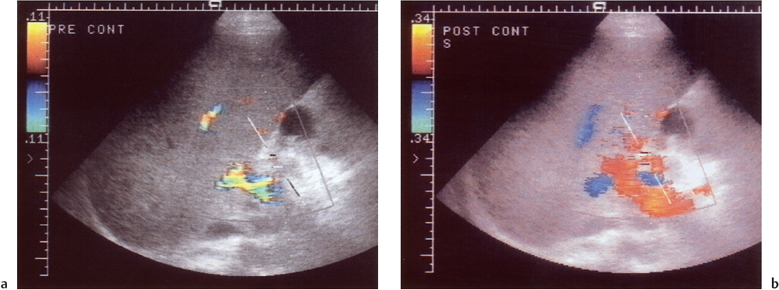
Fig. 10.6 Nonvisualization of any color Doppler signal from the hepatic artery (cursor) despite optimization of machine settings. b Following administration of microbubble contrast medium (Levovist), color Doppler signal fills the arteriallumen, allowing confirmation of a patent post-transplant hepatic artery
The normal Doppler waveform in the hepatic artery is of a broad systolic peak with high diastolic velocity and low RI. Any deviation from this pattern must be carefully watched and followed up with serial CDUS, with a low threshold for proceeding to arteriography, the gold standard in evaluation of hepatic artery patency. Although the hepatic arterial waveform in the immediate postoperative period varies, impending thrombosis is suggested when a previously normal Doppler waveform proceeds to absence of diastolic flow with dampening of the systolic peak. This is thought to occur prior to progression to complete loss of the hepatic arterial signal.5
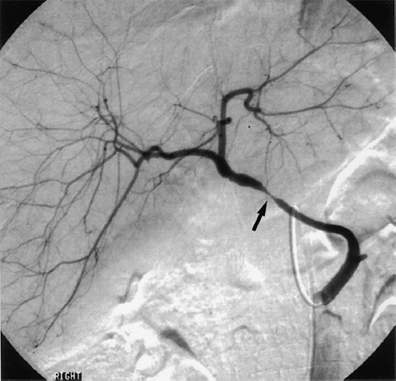
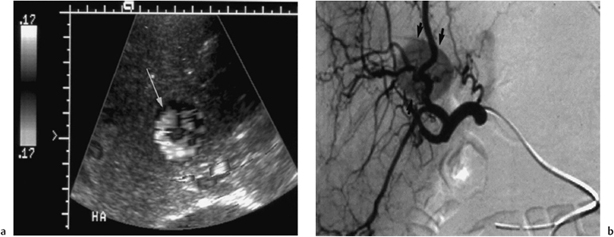
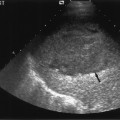
Fig. 10.7 Arteriography of a transplant hepatic artery demonstrates a narrowing of the artery at the surgical anastomotic site (arrow). (Reproduced from Clinical Radiology16 by permission of the Editor.)
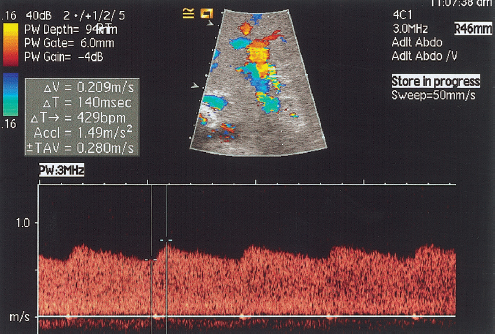
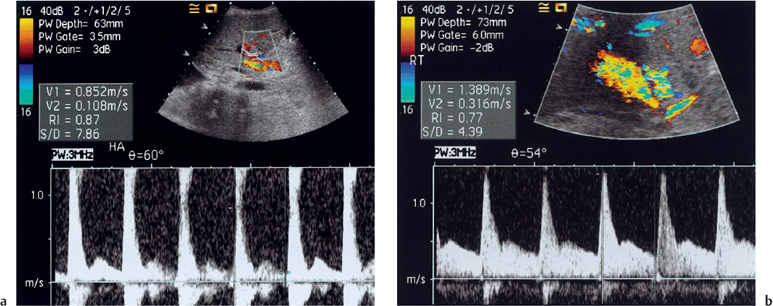
Fig. 10.8 Spectral Doppler waveform obtained from an intrahepatic section of the hepatic artery demonstrating the tardus parvus waveform (acceleration time 0.14 seconds, RI = 0.23)
With the advent of multislice capabilities, computed tomographic arteriography (CTA) of the liver is now a practical noninvasive method of detecting hepatic artery complications after liver transplantation. The excellent spatial resolution and fast scan times allow CTA to depict smaller vessels for evaluation of both patency and stenosis and may eventually replace arteriography.11 In addition, dual-phase contrast-enhanced three-dimensional magnetic resonance angiography (3D MRA) of the hepatic blood supply using fat-saturation techniques, the administration of a high caloric liquid prior to scanning, and digital image subtraction, can give comparable results to arteriography for the evaluation of hepatic arteries.12–14 Ultrasound will, however. continue to provide first-line imaging assessment of the post-transplant hepatic artery.
Hepatic Artery Stenosis
Stenosis of the hepatic artery has been reported to occur in up to 5 % of hepatic transplant recipients,15 but in our experience the incidence is close to 3%.16 Hepatic artery stenosis (HAS) commonly occurs in the early postoperative period, but may do so several years after transplantation; it occurs most often at the site of the surgical anastomosis (Fig. 10.7). If untreated, severe stenosis can lead to allograft rejection and progress further to the development of all the complications associated with HAT.17
HAS after liver transplantation may be attributable to inadequate surgical technique, surgical clamp injury, allograft rejection, microvascular injury associated with disrupted vasa vasorum, underlying liver disease, or preservation injury.15 HAS produces an intrahepatic spectral Doppler tardus parvus waveform, which is defined as a reduced RI of less than 0.5, and a prolonged systolic acceleration time (SAT) greater than or equal to 0.08 seconds (Fig. 10.8).18 Based on the tardus parvus waveform, sensitivity of between 85% and 97% for CDUS in detecting the presence of HAS has been quoted.15,18 However, if only one of the defining parameters—reduced RI or prolonged SAT—is present, the frequency of finding HAS is decreased.18
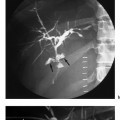
The tardus parvus spectral waveform is nonspecific for stenosis: similar patterns may be seen in severe aortoceliac atherosclerotic disease, arteriovenous fistula, arteriobiliary fistula formation, and long-standing thrombosis with collateral development.6 Therefore, the primary Doppler ultrasound criterion of arterial stenosis in any location of the body—a marked focal increase in peak systolic velocity (2 m/s) should be sought and is dependent on the direct evaluation of the site of the stenosis. In OLT, poor ultrasound evaluation of the vessels, particularly in the subhepatic space, markedly reduces the possibility of detecting a raised peak systolic velocity.6 The use of ultrasound micro-bubble contrast medium may be beneficial in patients with HAS (Fig. 10.9). Microbubble contrast enhances the Doppler and color signal by 20 dB, making it easier accurately to identify the exact site of the stenosis, and to identify collateral vessel formation if present.6 There are two situations in which the diagnosis of HAS should be made with caution: during surgery, using intraoperative Doppler ultrasonography; and in the early postoperative period (up to 48 hours). Frequently, the postoperative RI will over time return to normal in these patients. Therefore follow-up CDUS is recommended when a low RI is detected in the hepatic artery in the presence of normal hepatic function in the postoperative period.18 If clinical suspicion of HAS remains high, a normal CDUS examination should not prevent follow-up angiography, as HAS may not manifest the tardus parvus Doppler ultrasound abnormality.18 In the absence of a tardus parvus waveform the HAS is less severe, but nevertheless important to identify, in order that appropriate clinical management is undertaken.
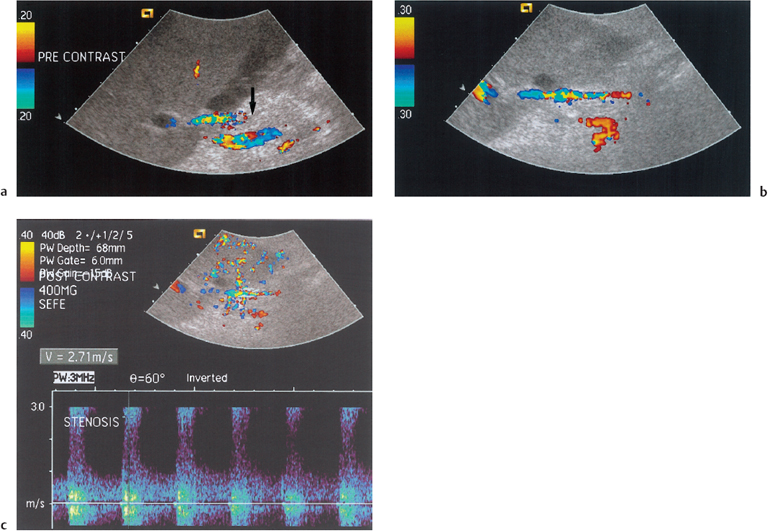
Fig. 10.9 a Incomplete color Doppler signal from the hepatic artery in the subhepatic space (arrow) in a post-transplant patient. b Following administration of microbubble contrast medium (Levovist) the hepatic artery fills with color Doppler signal. c Interrogation of the hepatic artery with spectral Doppler imaging reveals an area of increased velocity corresponding to a focal hepatic artery stenosis. (Reproduced from Clinical Radiology16 by permission of the Editor)