Key points
- •
Imaging of the postoperative spine and associated complications is a common clinical scenario for the radiologist; however, despite advanced imaging techniques and better spatial resolution, this remains a challenging task.
- •
Evaluation of the postoperative spine requires a general knowledge of the surgical approach to each spinal region and the normal temporal evolution of expected postoperative changes.
- •
Knowledge of specific complications relating to each surgical approach and an understanding of general complications common among various surgical procedures, such as the appropriate evaluation of postoperative hardware, will assist the radiologist in interpretation of postoperative fusion and complications.
Introduction
Back pain is one of the most common clinical complaints in medicine. Low back pain affects up to 80% of the population during their lifetime and 1% to 2% of the United States population is disabled by low back pain. In 2002 the National Health Interview Study sampled 36,161 households, and found that back pain within the last 3 months was the most frequent type of pain reported with 26.4% of the respondents. The rate of spine surgery in the United States is the highest in the world, more than 1.2 million spinal surgeries being performed each year, although there is high geographic variation suggesting poor professional consensus on treatment approaches. For example, Medicare data for 2001 show a 6-fold variation in spine surgery rates among United States cities and a 10-fold variation in the rate of spinal fusion.
Postoperative complications may be immediately apparent after surgery or delayed by weeks, months, or years. The overall incidence of major neurologic deficit immediately after spinal surgery is low, with an overall incidence of less than 1%, and slightly more common after thoracic spine surgery (0.49%), followed by the cervical (0.29%) and lumbar spine, respectively (0.08%). The etiology of major neurologic injury during spinal surgery can include direct surgical trauma to the cord or neural elements, compression and/or distraction of the vertebral column, vascular compromise (local infarct or systemic hypotension), epidural and subdural hematoma, and mechanical compression from infolding of the ligamentum flavum, posterior longitudinal ligament, disc, or adjacent bony structures. Over time an estimated 10% to 20% of patients will experience 1 or more complications relating to surgery, and imaging plays an important role in preoperative assessment and postoperative management.
Routine scheduled postoperative imaging may be performed in otherwise asymptomatic patients to evaluate the position and appearance of spinal instrumentation or to assess the progression of spinal fusion. Alternatively, imaging is often performed to assess immediate or delayed postoperative complications in the symptomatic patient and to further evaluate patients with little or no relief of symptoms following the initial surgical procedure; the so-called failed back surgery syndrome.
Introduction
Back pain is one of the most common clinical complaints in medicine. Low back pain affects up to 80% of the population during their lifetime and 1% to 2% of the United States population is disabled by low back pain. In 2002 the National Health Interview Study sampled 36,161 households, and found that back pain within the last 3 months was the most frequent type of pain reported with 26.4% of the respondents. The rate of spine surgery in the United States is the highest in the world, more than 1.2 million spinal surgeries being performed each year, although there is high geographic variation suggesting poor professional consensus on treatment approaches. For example, Medicare data for 2001 show a 6-fold variation in spine surgery rates among United States cities and a 10-fold variation in the rate of spinal fusion.
Postoperative complications may be immediately apparent after surgery or delayed by weeks, months, or years. The overall incidence of major neurologic deficit immediately after spinal surgery is low, with an overall incidence of less than 1%, and slightly more common after thoracic spine surgery (0.49%), followed by the cervical (0.29%) and lumbar spine, respectively (0.08%). The etiology of major neurologic injury during spinal surgery can include direct surgical trauma to the cord or neural elements, compression and/or distraction of the vertebral column, vascular compromise (local infarct or systemic hypotension), epidural and subdural hematoma, and mechanical compression from infolding of the ligamentum flavum, posterior longitudinal ligament, disc, or adjacent bony structures. Over time an estimated 10% to 20% of patients will experience 1 or more complications relating to surgery, and imaging plays an important role in preoperative assessment and postoperative management.
Routine scheduled postoperative imaging may be performed in otherwise asymptomatic patients to evaluate the position and appearance of spinal instrumentation or to assess the progression of spinal fusion. Alternatively, imaging is often performed to assess immediate or delayed postoperative complications in the symptomatic patient and to further evaluate patients with little or no relief of symptoms following the initial surgical procedure; the so-called failed back surgery syndrome.
Surgical approaches and specific related complications
Multiple surgical techniques are available to access the spinal osseous elements, spinal cord, nerve roots, and intervertebral disc. The individual techniques may vary by region and underlying abnormality. However, the basic goal of each technique is similar: to facilitate safe access to the pathologic area of interest and minimize potential risk. In general, spinal surgeries can be categorized as decompressive or spinal stabilization/fusion procedures, although many examples are a combination of both. Decompressive procedures include discectomy, laminotomy, laminectomy, and facetectomy to decompress a stenotic spinal canal or neural foramen. Spinal fusion allows stabilization of spinal segments for developmental, degenerative, and posttraumatic instability, or iatrogenic causes of instability such as prior surgery.
Cervical Spine Approaches
Anterior cervical approach
The transoral-transpharyngeal approach allows access to the anterior clivus, C1, and C2 for a variety of abnormalities including basilar invagination, odontoid fractures or nonunion, rheumatoid arthritis with cranial settling and/or pannus formation, developmental disorders such as os odontoidium and odontoid hypoplasia, and tumors. The alternative approach to the anterior spine is via a retropharyngeal technique. The anteromedial approach uses the space between the carotid sheath laterally and the sternocleidomastoid muscle and tracheoesophageal complex medially, whereas an anterolateral approach goes lateral to the carotid sheath.
Although rare, occurring in less than 0.2% of anterior cervical discectomy and fusion (ACDF) surgeries, one of the most feared complications related to the anterior cervical approach is direct spinal cord injury. Spinal cord impingement or direct spinal cord trauma may result from vertebral body screws that extend too far through the posterior cortex. However, the risk of spinal cord injury is higher when instruments are advanced into the spinal canal from the anterior approach to remove posterior osteophytes. Plain radiographs and/or computed tomography (CT) will show misplacement of screws or other hardware that impinge or traverse the spinal cord. On magnetic resonance (MR) imaging spinal cord contusion or laceration is manifest, with focal areas of T2 hyperintensity within the spinal cord. If there is associated hemorrhage, gradient recalled echo sequences may show foci of susceptibility within the spinal cord.
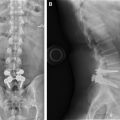
The most common reported complications associated with anterior cervical approaches include postoperative dysphagia, postoperative hematoma, and recurrent laryngeal nerve palsy. Dural leak and esophageal perforation are additional less common, but clinically important complications related to the anterior cervical approach. Although postoperative dysphagia is common, imaging of the neck is often normal unless there is clear evidence of hardware failure or malpositioning that results in impingement on the esophagus and adjacent soft tissues ( Fig. 1 ). A barium esophagram may show luminal narrowing during dynamic visualization of swallowing or delayed transit.
Injury to the vertebral artery, estimated to occur in 0.25% of anterior cervical discectomies, may occur following removal of bone too far laterally or lateral placement of hardware. The location of cervical hardware is best evaluated on CT. If vertebral artery injury is suspected intraoperatively or the patient presents with new neurologic deficits, initial evaluation with CT angiography will allow direct evaluation of hardware positioning and evaluation for potential vascular injury. There may be localized irregularity with or without narrowing, or demonstration of a dissection flap. Long tapered narrowing or occlusion is highly suggestive of dissection. Alternatively, vascular imaging may show focal pseudoaneurysm or active contrast extravasation.
Posterior cervical approach
A posterior approach to the cervical spine allows direct access to the posterior elements and the potential for wide exposure. A laminotomy, laminectomy, or laminoplasty can be performed to treat multilevel degenerative spondylosis, soft disc herniation, and ossification of the posterior longitudinal ligament.
The major complications associated with the posterior cervical approach include new transient or permanent cervical radiculopathy, vertebral artery injury, and progressive postlaminectomy kyphosis.
The placement of posterior fusion hardware in the cervical spine differs from that in the thoracic and lumbar spine. The pedicles in the cervical spine are small from C3 to C6 and larger at C2 and C7. Therefore, posterior cervical fusion typically involves lateral mass screws from C3 to C6, with traditional pedicle screws being reserved for the larger C2 and C7 levels. Direct impingement by osseous fragments or malposition of spinal hardware may result in new cervical radiculopathy, best visualized on CT. Other causes of new postoperative radiculopathy, including distraction of neural elements, direct trauma during surgery, and symptomatic hematoma, may require MR imaging for visualization. The rate of vertebral artery injury with the posterior cervical approach is less than 1% and is usually secondary to malposition of lateral mass or pedicle screws.
The rate of postoperative kyphosis varies widely, although it is clearly higher in patients who do not undergo fusion and those with spinal cord dysfunction. Kyphosis is easily evaluated with radiographs, and flexion-extension views may also be helpful to determine the degree of physiologic dysfunction.
Thoracic Approaches
Thoracic disc herniations represent less than 1% of symptomatic spinal disc herniations, although there is a high incidence of incidental disc herniations visualized on MR imaging (>15%). Unlike cervical and lumbar laminectomy, there is a relatively high incidence of neurologic injury with a posterior laminectomy in the thoracic spine and, therefore, procedures are designed to approach the pathologic region as directly as possible with minimal manipulation of the spinal cord. In general, approaches to the thoracic spine are considered anterior and posterolateral. Anterior approaches include a variety of transthoracic procedures as well as the thoracoscopic approach. Posterior and posterolateral approaches include transpedicular (with or without endoscopy), transfacet, and transforaminal, in addition to costotransversectomy and lateral extracavitary approaches.
Intraoperative localization of thoracic vertebral levels remains a specific concern in thoracic spine surgery. Indeed, a 2008 questionnaire study found that nearly 50% of spine surgeons surveyed reported a wrong level surgery during their career. A variety of factors including overlying scapular shadows, variation in the number of rib-bearing vertebrae, and osteopenia all contribute to the difficulty in accurately localizing specific thoracic vertebral levels intraoperatively. To avoid this potential complication, preoperative placement of radiopaque markers using fluoroscopy or CT at the pedicle of interest may be helpful.
Lumbar Approaches
Anterior lumbar approaches
Anterior lumbar interbody fusion (ALIF) is typically performed when posterior decompression is not required and pain is the predominant symptom. With a lower abdominal or retroperitoneal approach through the flank, disc material is removed and disc height restored with interbody spacers. Stabilization is most commonly supplemented with an anterior metallic plate and vertebral screws, although occasionally lateral or posterior instrumentation is used. Complications specific to the anterior lumbar approach include vascular injury and retrograde ejaculation. The incidence of vascular complications shows a variable range in the literature, although a recent review suggests an incidence of less than 5%. Overall, vascular injury is more common during laparoscopic procedures than during open ALIF, and venous laceration is more common than arterial injury. Visceral injury such as bowel perforation is rare. Retrograde ejaculation is associated with manipulation of the autonomic plexus, which is closely associated with the aorta and vena cava at the bifurcation, and drapes over the ventral surface of the spine and sacral body. Rates of retrograde ejaculation vary up to 7.2% in the literature, with higher rates noted in cases using human recombinant bone morphogenic protein 2.
Posterior lumbar approaches
Posterior lumbar discectomy without fusion is used to decompress the disc space for treatment of disc herniation. The standard open discectomy involves laminotomy or hemilaminectomy, resection of the ligamentum flavum, and retraction of neural elements, allowing access to the disc space and excision of disc material.
Posterior lumbar interbody fusion (PLIF) involves wide bilateral laminectomies and partial facetectomy. With distraction of the vertebral segment, discectomy is performed and restoration of disc height is achieved with bone graft (autologous or allograft) or synthetic materials such as polymer cages or metal. A common variation of PLIF is transforaminal lumbar interbody fusion (TLIF) with a posterolateral approach through the foramen. Resection of the ipsilateral facet allows access to the thecal sac and disc.
Posterolateral fusion (PLF) is an alternative or supplement to PLIF whereby bone graft is placed laterally between the transverse processes rather than between the vertebral bodies. PLF is used primarily when there is severe loss of disc height, and insertion of an interbody spacer may result in neurologic compromise. PLF is usually supplemented by posterior instrumentation such as pedicle screws and stabilization rods.
Lateral and axial interbody fusion
Extreme lateral interbody fusion and axial interbody fusion have also been described. This far lateral approach places the lumbosacral plexus at risk of injury, as well as the genitofemoral nerve that lies in the psoas muscle. The most common complication associated with this approach is anterior thigh pain and weakness of hip flexors.
Complications of hardware devices and instrumentation
A familiarity with the commonly used devices and instrumentation used in the spine is important for the evaluation of the adequacy of hardware positioning and the assessment of complications such as hardware failure, device migration, or loosening.
It is important to stress that the instrumentation used in spinal fusion is used as an adjunct to bony fusion, to decrease the incidence of pseudarthrosis and to stabilize the spine until osseous fusion is complete. Without bony fusion, all devices will eventually fail under physiologic mechanical stress.
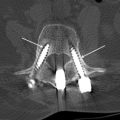
Hardware failure results when an implant breaks or is displaced relative to the underlying bone. Fracture of hardware components without significant displacement is best visualized on CT or plain radiographs, as MR imaging is limited because of hardware artifact. Before device failure there is often a preceding phase of loosening related to instability from ongoing motion, pseudarthrosis, or infection. Loosening appears as a rim of lucency surrounding screws or other devices, particularly when this lucency exceeds 2 mm or increases on sequential examinations ( Fig. 2 ).
Anterior fixation devices typically consist of an anterior plate anchored to the vertebral body with screws. The plate should be flush with the anterior vertebral body. Screws pass through the anterior cortex and are seated in the posterior cortex without cortical breakthrough. Ideally screws should not enter an adjacent endplate. Screws that extend too far through the posterior cortex may impinge or damage the spinal cord. Other complications include screws fracturing under stress or backing out of the plate, although many newer constructs include a locking plate with special cannulated screws, and screw caps that lock the plate and screws together preventing the screws from backing out.
As with hardware in other anatomic areas, there can be irritation or impingement on the adjacent soft tissues. In the cervical spine this may cause prevertebral or retropharyngeal inflammation or injury of vascular structures, esophagus, or trachea.
Pedicle screws in the thoracolumbar spine and lateral mass screws in the subaxial cervical spine are commonly used in combination with plates, hooks, or rods for spinal fusion. This approach results in a strong fusion construct and high rates of spinal fusion. The location of pedicle screws and their close proximity to adjacent neural and vascular structures results in a reported complication rate of 2.4% per screw. Optimal screw placement is along the medial aspect of the pedicle without breaching the cortex, entering the neural foramen or extending beyond the vertebral body cortex ( Fig. 3 ). The most common reported complication of pedicle screws is medial angulation of the screw with violation of the medial cortex, resulting in irritation of nerve root.
Interbody fusion may be performed with morselized autografts placed within and/or around bone graft or interbody fusion cages. Although many interbody fusion devices are radiolucent, they can be visualized on radiographs and CT with small radiopaque markers that are contained within the device. The posterior marker should be at least 2 mm anterior to the posterior vertebral body margin to exclude ramp/cage protrusion into the spinal canal.
Total disc replacement (TDR) is performed for discogenic pain without significant spinal stenosis or spondylolisthesis, and has become an increasingly popular alternative to spinal fusion. TDR is used to restore normal mobility and decrease the incidence of accelerated degenerative changes to adjacent segments. As with other new devices, there is a relative paucity of information regarding the complications associated with total disc replacement surgery. A recent review describes an overall complication rate related to the surgical approach (eg, vascular or nerve root injury, retrograde ejaculation) of approximately 2.1% to 18.7% and complications related to the prosthesis (such as migration or displacement, subsidence, implant failure, and endplate fractures) ranging from 2.0% to 39.3%.
As an alternative to traditional decompression, interspinous spacer devices have recently been introduced. These spacers are inserted between the spinous process through a small incision along the interspinous ligament, and function to reduce spinal stenosis by restoring height posteriorly, reducing infolding of the ligamentum flavum and overriding facets. The appearance of specific devices varies slightly, and there may be a hollow component within the spacer for placement of bone graft material, as well as fusion spikes along the wings of the device for attachment to the adjacent spinous process above and below. Complications include posterior migration or extrusion of the device, erosion into the adjacent spinous process, or spinous process fracture that may occur with distraction of the vertebral segment during insertion or during the postoperative course.
Pseudarthrosis
Failure of progression to solid osseous fusion is often the result of ongoing motion. The rate of pseudarthrosis has been reported to be as high as 20% in some series, and risk factors include revision surgery for prior nonunion, chronic illness, smoking, and long-term use of nonsteroidal anti-inflammatory drugs (NSAIDs). In particular, smoking has been shown experimentally to inhibit revascularization and suppress bone formation, and multiple studies have shown increased rates of nonunion in fusion of the cervical and lumbar spine, although the effect is diminished with the use of rigid instrumentation. The presence of pseudarthrosis itself may be symptomatic with ongoing microscopic or macroscopic motion, further contributing to degenerative changes. Increased stress on mechanical hardware can ultimately result in failure. Osseous fusion is best demonstrated on serial radiographic assessment or CT, and evaluation is based on the assessment of multiple factors ( Box 1 ).
- •
Visible bone formation bridging the level of fusion
- •
No clear lucency traversing the fusion or graft material
- •
No motion or less than 3° of change in flexion and extension views
- •
No periprosthetic lucency or halo
- •
No reactive sclerosis in the graft or adjacent vertebrae
- •
No fracture of the device, graft, or vertebrae
Postoperative infection
The incidence of infection following spinal surgery is low. However, with the increasing number of total spine surgeries, this is a relatively common indication for imaging of the spine. The use of spinal instrumentation clearly increases the risk for postoperative infections, and infection in the presence of implants is a major diagnostic and therapeutic challenge for surgeons and radiologists. Postoperative infection may occur in the immediate postoperative course or as a late complication, particularly in the setting of spinal instrumentation. Early postoperative bacterial spondylodiscitis likely occurs as a result of direct contamination. Late-onset infections may be secondary to hematogenous seeding, or the sterile inflammatory response around hardware components may stimulate low-virulence organisms to fester.
A recent review of surgical-site infections suggests that the average incidence is approximately 1% after simple discectomy. The risk increases with the presence of spinal instrumentation and predisposing factors such as chronic medical illness (renal failure, diabetes mellitus), intravenous drug use, and immunocompromised states, with an overall incidence between 0.7% and 12%.
In the postoperative setting, enhancement of lumbar nerve roots may be seen in up to 20% of patients with no recurrent clinical symptoms up to 6 weeks following surgery, but in only 2% of asymptomatic patients at 6 months. Thus the appearance of nerve root enhancement in this setting presumably represents a transient sterile radiculitis. Therefore, nerve root enhancement does not necessarily imply meningitis, and must be interpreted in context with surgical timing and other clinical factors. In addition to nerve root enhancement, imaging signs of meningitis in the spine include vague increased cerebrospinal fluid (CSF) signal intensity, resulting in an indistinct appearance of the spinal cord–CSF interface. There may be clumping of nerve roots and, in severe cases, obliteration of the subarachnoid space with purulent material. Spinal cord signal intensity is often normal; however, T2-weighted/short-tau inversion recovery sequences may show focal and/or diffuse areas of hyperintensity within the spinal cord. Following the intravenous administration of a gadolinium contrast agent, there is leptomeningeal and nerve root enhancement that may be smooth or nodular. There may also be linear or nodular enhancement within the subarachnoid space, related to septations or enhancing inflammatory products and debris.
Normal postoperative changes within the intervertebral disc in asymptomatic patients following discectomy include edematous changes within the vertebral endplates and endplate enhancement. Postoperative enhancement of the endplate is visualized as 2 linear bands paralleling the endplates with or without increased T2 signal intensity within the disc space. These changes at the site of discectomy and at the peridural and paraspinal soft tissues can be indistinguishable from infection in the postoperative setting and, therefore, correlation with clinical symptoms and laboratory markers such as white blood cell count, erythrocyte sedimentation rate, and C-reactive protein. In specific instances an image-guided percutaneous aspiration and/or biopsy may be required.
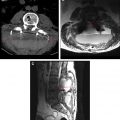
Imaging features that are suggestive of discitis/osteomyelitis include disc space height loss, endplate erosion, and vertebral destruction on radiographs and CT. Imaging of advanced cases shows collapse of the vertebral body. On MR imaging, suspicious features include low T1-weighted signal intensity centered about the intervertebral disc involving the adjacent vertebral bodies with corresponding high T2 signal intensity. There may be diffuse or rim contrast enhancement of the disc space and avid contrast enhancement of the adjacent vertebral marrow. A paraspinal and/or epidural phlegmon or abscess may also be present. This combination of MR imaging findings within the disc space, adjacent vertebral bodies, and paravertebral soft tissues is strongly suggestive of spondylodiscitis ( Figs. 4–6 ).