Fig. 20.1
Maternal mortality ratio worldwide in 2010. Courtesy: World Health Organization (reprinted with permission)
Similarly, perinatal and neonatal mortality rates remain unacceptably high in LICs with a majority of these deaths occurring in five countries: India, Nigeria, China, Pakistan and Democratic Republic of Congo. Although global perinatal mortality rates have decreased by 28 % between 1990 and 2009, experts believe this reduction is not sufficient to reach the target for MDG 4 by 2015 [6, 7].
The leading causes of maternal mortality are hemorrhage, infection, eclampsia, and obstructed labor; for perinatal mortality, the leading causes are infection, birth injuries, preterm births, and birth defects [3, 8]. These deaths are largely preventable and a number of publications have discussed potential strategies for intervention: increasing access to emergency obstetric services, increasing availability of skilled health care providers, increasing access to antibiotic treatment, employing safe abortion practices, and promoting disease prevention through hygiene-focused health education [9–11].
Although the utility of ultrasound imaging in LICs has not been extensively studied, experience from the developed world suggests that this technology may play a key role in early diagnosis and management of common maternal-fetal conditions. This review will address the potential value of ultrasound for the management of these conditions in developing countries.
Role of Imaging
Ultrasound is currently the most widely employed imaging modality for evaluating fetal well-being during pregnancy [12–14]. While access to sonography may be limited in developing countries, the recent introduction of compact, portable and affordable units may allow more widespread use of this technology [15]. The most widely available compact units are manufactured by two companies: GE Healthcare (Milwaukee, WI) and Sonosite, Inc. (Bothell, WA); other global manufacturers are not far behind, producing their own low-cost machines. These small units are sturdy, portable, and offer unique features such as a rechargeable or solar battery, which are well-suited for areas with unreliable electricity. Most of the currently-offered compact machines lack the more advanced features of full-sized units such as color Doppler technology or high resolution transducers. There is little scientific data on whether these features are necessary for detection of life-threatening obstetric complications in low resource settings; furthermore, there are few studies that compare the accuracy of full-sized ultrasound units with compact machines in obstetric imaging [15]. However, there is some early evidence that compact machines may provide enough information to determine appropriate triage for patients with obstetric emergencies [16].
The impact of imaging on maternal-fetal outcomes in developing countries remains an ongoing area of research, and currently very few controlled trials on this subject are available for review. However, knowing the causes of morbidity and mortality, inference of potential roles of imaging can be made. The impact of obstetric ultrasound may be seen in conditions specific to the mother, fetus, and a few syndromes when fetal illness affects maternal health.
Causes of Maternal Mortality
Maternal Hemorrhage
Ectopic Pregnancy
An ectopic pregnancy, or eccysis, is a complication of pregnancy in which the embryo implants outside the uterine cavity. With rare exceptions, ectopic pregnancies are not viable. Furthermore, they are dangerous for the mother, since internal hemorrhage is a life-threatening complication. Hemorrhage secondary to ruptured ectopic pregnancy is an important and preventable cause of early pregnancy-related deaths in both HICs and LICs. Clinical diagnosis of ectopic pregnancy can be difficult as the typical signs and symptoms of irregular vaginal bleeding (tender palpable adnexal mass, and abdominal or pelvic pain) may not be present. In patients presenting with a positive pregnancy test and non-specific signs and symptoms, ultrasound can be very useful in confirming or excluding an ectopic pregnancy. Typical sonographic findings in ectopic pregnancy include: an adnexal mass containing a yolk sac or embryo, adnexal mass without an intrauterine gestational sac (Fig. 20.2), large amount of free cul-de-sac fluid, or presence of a normal gestational sac in an ectopic location such as the uterine cornua or cervix (Fig. 20.3). Early diagnosis may potentially allow urgent surgical intervention, thereby averting serious complications including death upon rupture.
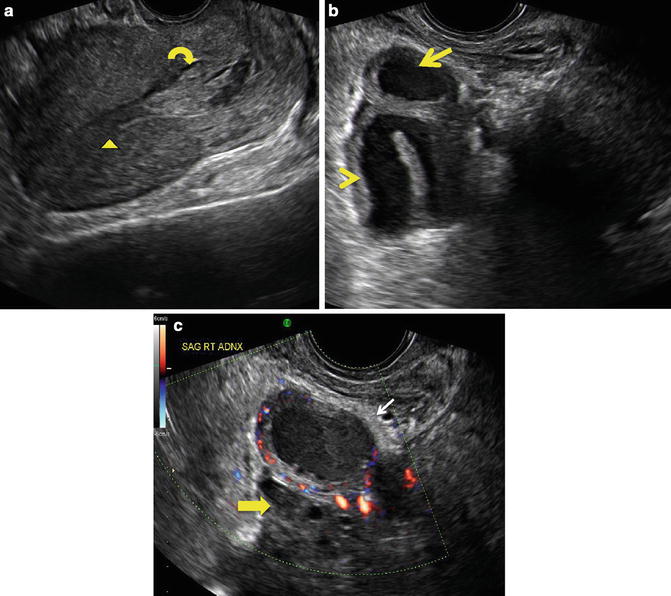
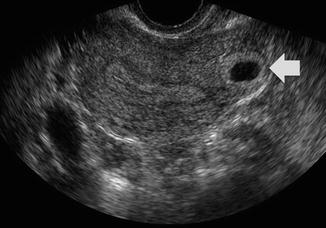

Fig. 20.2
(a) Sagittal midline view of the uterus on endovaginal ultrasound performed at 10 weeks gestation by clinical dates. There is a thin endometrial stripe (arrowhead) in uterus without gestational sac. Note blood products in the lower uterine segment and cervix (curved arrow). (b) Sagittal imaging in the right adnexa in the same patient demonstrates a thickened, dilated fallopian tube (arrowhead). Arrow demonstrates a mass at the end of the fallopian tube, highly suspicious for a tubal ectopic pregnancy. (c) Sagittal Doppler imaging of the right adnexa in the same patient demonstrates further detail of the adnexal mass (white arrow) with peripheral blood flow (ring of fire). Note that this mass is separate from a normal ovary seen just inferior to it (yellow arrow). (Courtesy S. Ghate, Author)

Fig. 20.3
Endovaginal ultrasound of another patient with positive beta-HCG. Transverse view of the superior uterus shows an eccentric implantation of the gestational sac in the uterine fundus with minimal myometrium surrounding it (wide arrow). The ovaries were normal (not shown). Findings are consistent with cornual ectopic pregnancy. (Courtesy S. Ghate, Author)
While the incidence of ectopic pregnancy has increased in the United States, mortality from its complications has declined in recent years. Much of this decrease has been attributed to early detection by ultrasound imaging [17, 18]. Similarly in LIC, where ultrasound has been utilized, diagnosis of ectopic pregnancy prior to rupture and subsequent intervention has resulted in the reduction of maternal morbidity and mortality [19, 20].
Abnormalities of the Placenta and Cord
Implantation Abnormalities
Ultrasound evaluation of placental implantation can provide valuable information concerning maternal risk in pregnancy. The placenta normally attaches to an intact endometrial lining within the uterus. With scarring from previous Caesarian section, prior instrumentation, or trauma, the placenta may invade to (accreta), into (increta), or beyond (percreta) the endometrium. Mild degrees of invasion (accreta) may not produce clinical symptoms, but could result in marked intra- or post-partum hemorrhage with severe cases resulting in hysterectomy or maternal death. With prior knowledge of an implantation abnormality, strategies for delivery may be planned in order to avoid serious complications. Although MRI is most accurate at determining degree of invasion and detection of posterior placenta accreta, recent studies have shown that US is also reliable, detecting 50–80 % of placenta accrete [21]. Sonographic findings of loss of the retroplacental clear space and presence of multiple lacunar spaces within the placental body may be suggestive of an implantation abnormality (Fig. 20.4). For women who are at high risk for an implantation abnormality, US may be an effective, low cost alternative to MRI, particularly in developing countries where access to MRI may be limited [21].
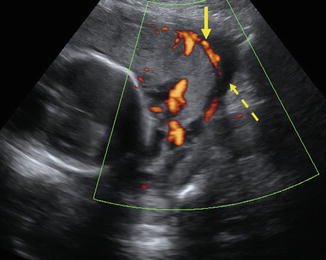

Fig. 20.4
32-year-old patient with history of prior Caesarian section, presented with hematuria. Color Doppler imaging demonstrates placenta (solid arrow) abutting the maternal bladder (dashed arrow) without intervening myometrium, suggesting invasion of the placenta to the level of the bladder wall. These findings are highly suggestive of placenta percreta. (Courtesy S. Ghate, Author)
Previa
Clinical symptoms of painless vaginal bleeding can be attributed to a number of causes including uterine fibroids, cervical polyps, placenta previa or vasa previa. Of these, both placenta previa and vasa previa may result in significant maternal or fetal blood loss during vaginal delivery. Placenta previa occurs when the placenta either extends to the margin of, partially covers, or completely covers the internal cervical os in the third trimester. With vasa previa, vessels from the fetal circulation may cross the internal cervical os (Fig. 20.5). During vaginal delivery, these vessels can tear and result in fetal blood loss. Vasa previa carries a fetal mortality risk of 33–100 % [22]. Early prenatal identification can avoid this outcome [22].
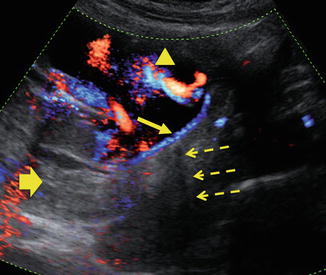

Fig. 20.5
25-year-old patient presented with painless vaginal bleeding. Color Doppler imaging of a gravid uterus at 20 weeks gestation demonstrates a posterior placenta (wide arrow) with velamentous insertion of the cord anteriorly (arrowhead). In addition, cord vessels (solid arrow) are seen crossing the internal cervical os, (dashed arrow) consistent with vasa previa. (Courtesy S. Ghate, Author)
Careful ultrasound imaging with or without Color Doppler can detect these abnormalities prenatally and guide the obstetrician to appropriate cesarean delivery planning.
Gestational Trophoblastic Disease
Gestational trophoblastic disease (GTD) describes a group of rare pregnancy-related trophoblastic neoplasms. Histologically, these tumors are classified as benign hydatiform moles, invasive mole, choriocarcinoma or placental site trophoblastic tumor (PSTT). Patients may present with hyperemesis, bleeding, enlarged uterus, and elevated serum beta-HCG levels. Ultrasound findings of an abnormal mass with multiple cystic spaces (Fig. 20.6) may help differentiate GTD from a multiple gestation pregnancy which may have similar signs and symptoms, but where clinical management will differ greatly.
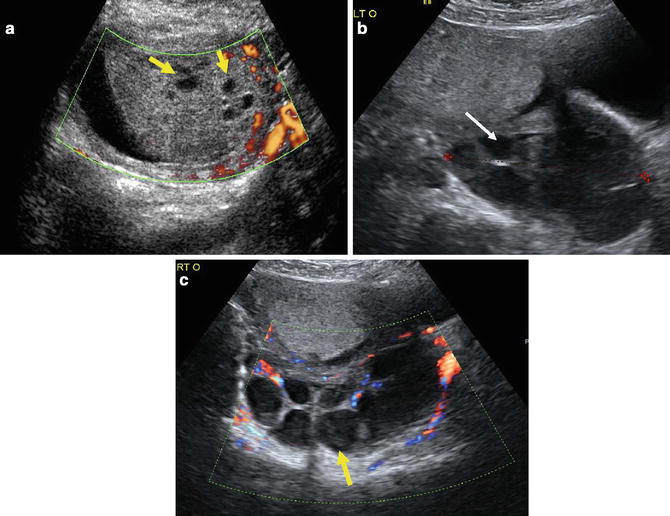

Fig. 20.6
(a) 32-year-old patient with a positive beta-HCG presented with symptoms of hyperemesis. This is a thickened placenta with multiple cystic spaces (arrows). No fetal tissue or oligohydramnios are present. (b, c) Images of the ovaries in the same patient shows multiple large cysts (arrows) consistent with theca luteal cysts. This constellation of findings is suspicious for a complete molar pregnancy. (Figure courtesy S. Ghate, Author)
Early identification of GTD by US and subsequent early surgical intervention may reduce the risk of maternal morbidity from severe hemorrhage, preeclampsia, thyrotoxicosis or ovarian hyperstimulation syndrome [23].
Abortion
The WHO estimates that approximately 13 % of maternal deaths are linked to unsafe abortions, from procedures performed by unskilled practitioners with or without the use of sterile technique [24, 25]. The most common complications from these procedures are hemorrhage and infection (sepsis). In these situations, ultrasound may be helpful in determining the causes of hemorrhage such as retained products of conception (POC) or incomplete abortions. Imaging findings suggestive of POC include gestational sac with or without an embryo, thickened or heterogeneous endometrial stripe with or without blood flow, or heterogeneous fluid collection within the endometrium. The use of ultrasound for definitive diagnosis may lead to earlier and more appropriate intervention for the condition. Timely detection and intervention are necessary as presence of POC can lead to sepsis, shock, hemorrhage, and maternal death [25–27].
In women who present with vaginal bleeding where a threatened spontaneous abortion is suspected, imaging may help differentiate nonviable from potentially viable pregnancies prior to surgical intervention. Ultrasound findings of irregular gestational sac without a yolk sac, absent cardiac activity, or too small gestational sac, can be diagnostic of a missed abortion or nonviable pregnancy (Fig. 20.7).
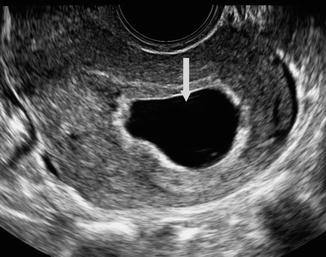

Fig. 20.7
Sagittal view of the uterus from endovaginal ultrasound performed for vaginal bleeding at 6 weeks and 5 days of gestation. Arrow denotes abnormally large, irregular gestational sac measuring 2.1 cm. No embryo or yolk sac is identified within the gestational sac. The findings are consistent with an anembryonic, nonviable pregnancy. (Figure courtesy S. Ghate, Author)
Maternal Sepsis
Maternal sepsis, an uncommon but important complication of pregnancy, is responsible for 10–12 % of maternal deaths in LICs [8, 26]. Clinical signs and symptoms of fever, chills, abdominal pain, or vaginal discharge in post-partum or post-cesarean section patients, may indicate infection resulting from endometritis, chorioamniotis, POC or abscess [27]. Early diagnosis, broad-spectrum antibiotic therapy and treatment of the underlying cause of sepsis may prevent serious complications such as maternal death, secondary infertility, or long-term morbidity from chronic pelvic pain [28]. A diagnosis of sepsis is generally made based on clinical presentation of the patient. While US may not be useful in diagnosis of sepsis, it may have value in detecting two important causes of sepsis: septic abortions or focal abscesses, both of which may require surgical intervention in addition to antibiotics for definitive treatment.
Causes of Perinatal Mortality
Fetal Growth Restriction
In women where menstrual history is unknown, such as with an unplanned pregnancy or failed contraception, first trimester crown rump length or second trimester biometry measurements provide an accurate and reliable estimate of gestational age as demonstrated by previous studies [29]. A precise estimation by sonography is necessary in order to avoid unnecessary preterm or post-date deliveries, which are important causes of perinatal mortality [30]. This accuracy decreases substantially by third trimester where measurements may have a much wider variation of normal. Accurate dating of a pregnancy theoretically allows early detection of fetal growth restriction (FGR), macrosomia, or post-date gestation and therefore, potentially facilitating timing and mode of delivery.
FGR is a complex diagnosis which may result in perinatal death from hypoxia, hypoglycemia, or meconium aspiration. There are two main types of FGR: asymmetric, from chronic fetal malnutrition, and symmetric, from decreased cellular growth. Asymmetric FGR is far more common and implicated in 90 % of cases [31]. While FGR may be suspected clinically, sonography may be more accurate in predicting causes of and confirming presence of a compromised fetus [32]. Sonographic findings suggestive of symmetric growth restriction include low estimated fetal weight (<10th percentile for gestational age), decreased fetal abdominal circumference, or oligohydraminos. Ultrasound findings of fetal structural anomalies may be predictive of an abnormal karyotype which may result in symmetric growth restriction, perinatal, or neonatal death. Other sonographic findings seen with both forms of growth restriction include abnormal umbilical artery cord Doppler ratios or waveforms, decreased fetal tone, movement or breathing. This diagnosis has implications for both maternal and fetal well-being. In the setting of preeclampsia/eclampsia, FGR is associated with severe placental disease and poor maternal and fetal outcome without appropriate treatment. Delivery is usually indicated in this context.
Abnormal Lie and Malpresentation
Malpresentation can cause birth injury, umbilical cord compression, or prolapse during delivery, potentially resulting in perinatal death. Ultrasound can be used as an adjunct to physical examination to confirm the presence of malpresentation and potentially guide the obstetrician to determine appropriate delivery planning.
Causes Affecting Both Maternal and Fetal Health
Multiple Gestation Pregnancies
Twin pregnancies carry a higher maternal risk of preterm delivery, post partum hemorrhage, preeclampsia, and eclampsia [33]. Perinatal mortality is also approximately five times higher in twin pregnancies when compared with singletons [34]. While multiple gestations can be detected by careful physical examination and Doppler fetal heart rate monitors, the chorionicity and amnionicity can only be detected by careful ultrasound evaluation. This allows identification of potential risk factors during pregnancy. Monochorionic twin pregnancies have roughly 2.5-fold increase in perinatal morbidity and mortality compared with dichorionic twins [35].
In the first or early second trimester, dichorionic-diamniotic pregnancies can be identified by the presence of a thick, dividing membrane, separate placentas, discordant genders, or presence of a “lambda” or “twin peak” sign [36, 37] (Fig. 20.8). Monochorionic twins share placental vessels, which may lead to shunting of blood from one twin to the other. This is often referred to as twin–twin transfusion syndrome (TTS), a complication seen in up to a 30 % of monochorionic gestations and best identified by US. TTS is a leading cause of fetal mortality. Early detection with careful follow-up and potential early delivery could lead to improved outcome [38].
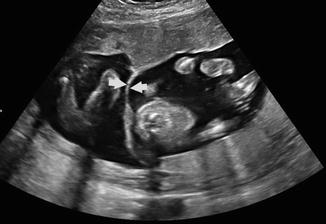

Fig. 20.8
Transverse view of twin pregnancy demonstrates a triangular peak of villi extending into the intertwin membrane consistent with “lamba” or “twin peak” sign in a dichorionic diamniotic pregnancy. (Figure courtesy M. Small, Author)
Hydrops Fetalis
The classic definition of hydrops fetalis is the abnormal presence of fluid in two or more compartments [39]. The most common ultrasound findings include polyhydramnios, fetal pleural effusions, ascites, pericardial effusions, hepatosplenomegaly and skin edema (Fig. 20.9). Sources of hydrops fetalis may be broadly categorized into maternal (immune) or fetal (non-immune) causes. Rhesus isoimmunization is the primary immune-mediated source of hydrops. Non-immune factors contributing to hydrops include: fetal cardiac anomalies, arrhythmias, chest masses, peripheral shunts resulting from fetal or placental tumors, aneuploidy, infection, or TTS in multiple gestation pregnancies. The prognosis for the fetus can be variable depending on the cause. In cases where Rh-incompatibility is the underlying cause, early detection by ultrasound imaging and follow-up with aggressive medical treatment, as well as close monitoring of the pregnancy and possible early delivery, can result in a favorable prognosis.
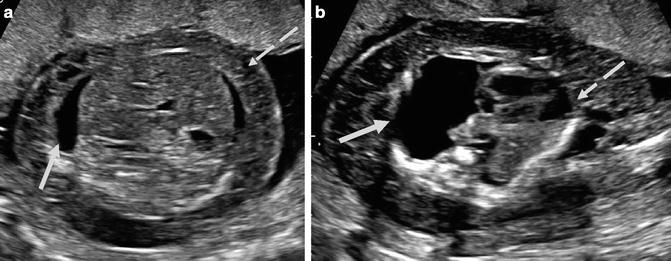

Fig. 20.9




(a) Axial view of the fetal abdomen demonstrates fetal ascites (solid arrow) and skin thickening (dashed arrow). (b) Axial imaging of the fetal thorax in the same patient demonstrates a large pleural effusion (thick arrow) with associated mass effect on the fetal heart (dashed arrow). (Figure courtesy S. Ghate, author)
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree