2 Prenatal sonographic diagnosis of congenital anomalies
Fetal ultrasound is now an established part of standard obstetric care in many countries. It can be performed in early gestation and in the first, second and third trimester (Table 2.1), as part of routine obstetric care, or as a targeted investigation in women at increased risk for a particular problem (Box 2.1). In many countries fetal ultrasound is offered routinely both in the first trimester of pregnancy to establish the gestation and viability and again later (around 20 weeks) to examine the anatomy in more detail. Scanning earlier and later in pregnancy in most units tends to be performed on clinical indication rather than routinely. In this chapter we will confine further discussion to the use of fetal ultrasound for the detection of congenital abnormalities and genetic syndromes in the first and second trimesters of pregnancy. All anatomical systems will be described, but the focus will be on those areas that present for postnatal ultrasonography.
BOX 2.1 Indications for detailed anomaly scanning
• Family history of abnormality/genetic condition
• Maternal medication (anti-epileptic, warfarin, lithium)
• Maternal recreational drug use
Risk factors developing in pregnancy
• Increased nuchal translucency on 10–14 week scan
• Raised maternal serum alfa-fetoprotein
• Abnormality detected on routine scanning
ICSI, intracytoplasmic sperm injection.
ROUTINE ULTRASOUND SCREENING FOR FETAL MALFORMATION
Despite the potential advantages of prenatal ultrasound examination, there is still much controversy about the value of ultrasound for routine fetal malformation screening. Studies report a considerable variation in the sensitivity, ranging from 20.7% to 82.4% in the first trimester (Table 2.2)1–6 and from 16.6% to 84.3% in the second trimester (Table 2.3).7–23 When interpreting this data it is important to differentiate between studies examining the use of routine fetal ultrasound as opposed to ultrasound used in a high-risk population where the investigation is targeted and detection rates are higher.24 Furthermore, the sensitivity of ultrasound screening varies according to the nature and severity of the malformation, the gestational age of the fetus, equipment used, fetal position, maternal body mass index and the experience of the ultrasonographer. Many of the studies reported (Tables 2.2 and 2.3) do not define these variables well. Finally, it must be remembered that the spectrum of abnormalities seen using prenatal ultrasound varies considerably from that seen postnatally. The prenatal sonographer will detect many lethal abnormalities (anencephaly, severe uropathies causing oligohydramnios and pulmonary hypoplasia, or complex cardiac anomalies) as well as anomalies that will be clinically silent in the newborn period and beyond (many renal anomalies, cystic lung lesions, or mild ventriculomegaly). The latter pose a difficult problem in defining optimum clinical management.
Table 2.2 Summary of studies reporting the detection of fetal abnormalities before 14 weeks using routine ultrasound
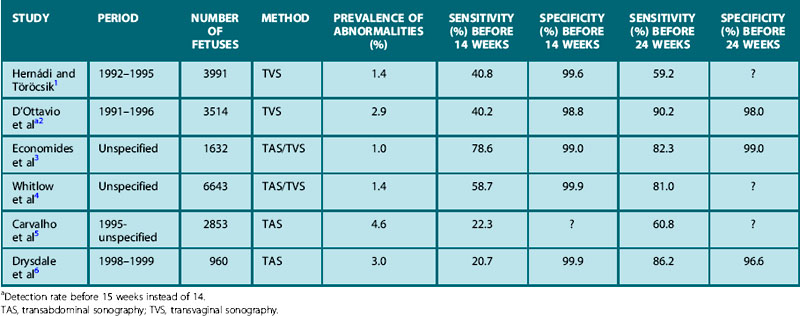
Table 2.3 Summary of studies reporting the detection of abnormalities before 24 weeks using routine ultrasound

The routine fetal anomaly scan
The Royal College of Obstetricians and Gynaecologists (RCOG) has issued guidelines for standards that should be applied for routine fetal anomaly scanning at 20 weeks’ gestation (Table 2.4).
Table 2.4 The routine fetal anomaly scan
| Structure | Features examined | |
|---|---|---|
| Routine anomaly scan | Extended views | |
| Head | Skull shape Ventricles, cerebellum, cavum septum pellucidum Measure circumference and diameter | |
| Face | Lips, nose | |
| Neck | Observe and measure nuchal fold | |
| Spine | Longitudinal and transverse views | |
| Heart | Four-chamber view | Left and right outflow tracts, aortic arch |
| Thorax | Diaphragm | |
| Abdomen | Stomach Cord insertion Kidneys and bladder Measure diameter | |
| Limbs | Three long bones in each limb Orientation of hands and feet Measure femur length | Count fingers and toes |
From Guidelines for a routine fetal anomaly scan. London: Royal College of Obstetricians and Gynaecologists; 1997, 2001.
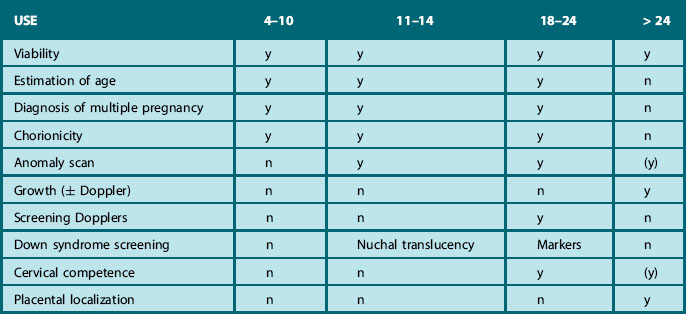
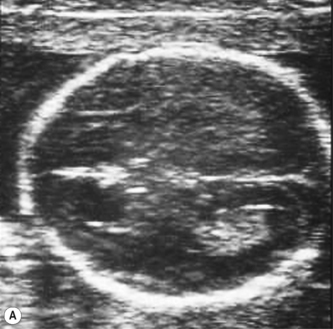
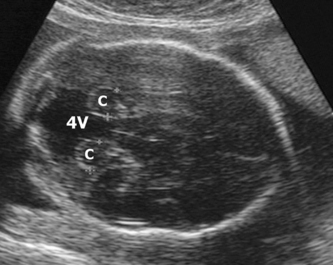
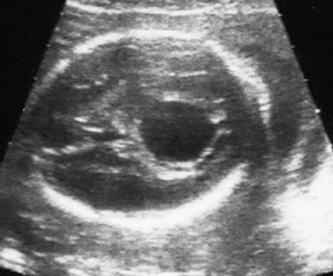
The head is examined in the axial plane, and the skull shape and mineralization are checked. Measurements of the circumference (HC) and the width of the anterior horns and posterior horns of the lateral ventricles should be performed at the level of the cavum septum pellucidum and the third ventricle (Fig. 2.1A). The sub-occipitobregmatic view will demonstrate the posterior fossa with the cerebellum and cisterna magna (Fig. 2.1B).
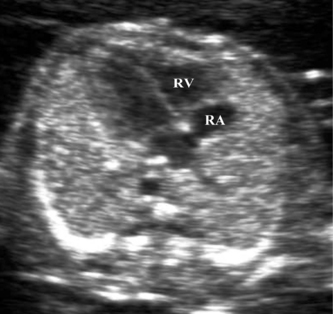
The thorax is examined both in the longitudinal and axial planes. In the longitudinal view the hemi-diaphragms can be identified, with the stomach lying below the diaphragm and the heart above (Fig. 2.2). In the axial plane the four-chamber view of the heart can be examined and should occupy one-third of the fetal chest (Fig. 2.3).
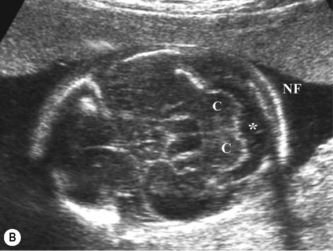
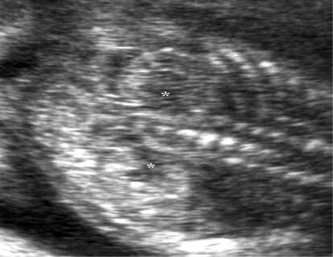
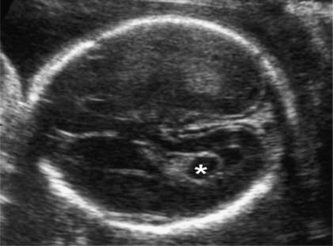
The abdomen is examined in the axial plane at the level of the stomach and the portal sinus of the liver for its shape and contents, and the circumference (AC) is measured at this level. A baseline AC measurement should be obtained in case growth failure is suspected later in pregnancy. The abdominal cord insertion is examined to exclude anterior abdominal wall defects. The kidneys (Fig. 2.4) and bladder are identified. The anteroposterior (AP) diameter of the renal pelvis should be measured and should normally be less than 5 mm. The perivesical arteries can be identified running around the bladder.
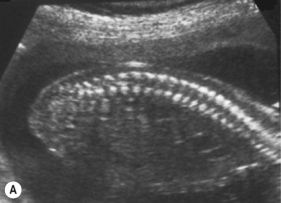
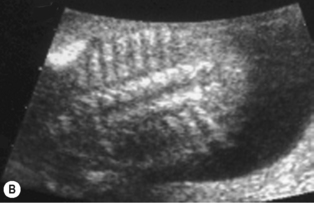
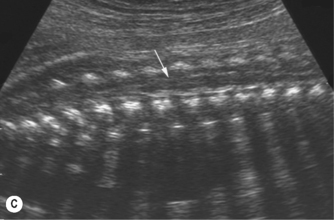
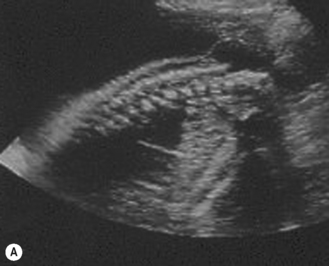
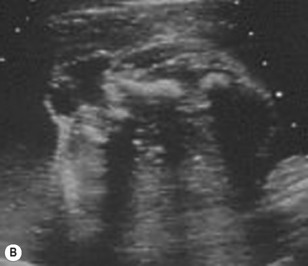
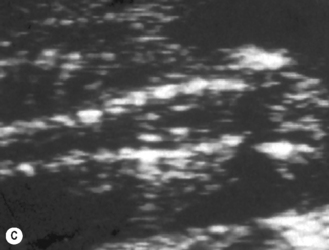
The spine should at the very least be examined in the sagittal and axial planes and, if possible, in the coronal plane (Fig. 2.5). In the axial plane the three ossification centers should be identified, with their skin covering, and should be seen to widen in the cervical region and narrow at the sacrum. In the sagittal plane (Fig. 2.5A) the normal curvature of the spine can be seen, with the upward sweep of the sacrum. Again the skin covering should be identified and the alignment of the laminae and vertebral bodies should be identified. In the coronal plane (Fig. 2.5B) the alignment of the transverse processes and laminae give a railway track appearance of the spine, which widens at the head and narrows towards the sacrum. In this view the alignment of the ribs can also be seen. With increasing resolution of modern ultrasound machines the spinal cord and cauda equina can be demonstrated (Fig. 2.5C).
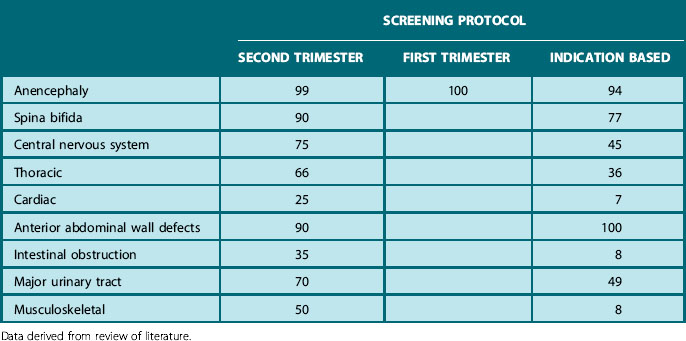
Finally, fetal gender can be examined and, when requested, revealed to the parents. Using this protocol around 80–85% of severely handicapping or lethal abnormalities and varying proportions of other anomalies should be detected (Table 2.5).
Table 2.5 Rate of detection (%) of fetal abnormalities before 24 weeks’ gestation, using different screening protocols
Ultrasound markers of fetal aneuploidy
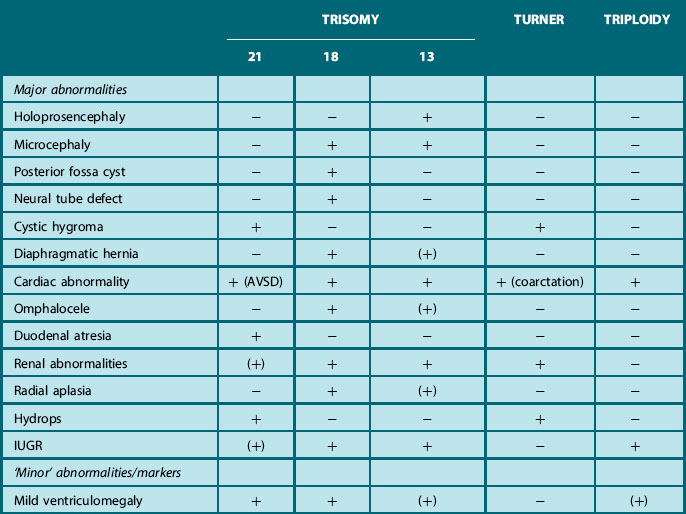
One group of cases that may not present to the postnatal sonographer are those associated with major chromosomal anomalies, since many of these conditions are lethal. In view of the high incidence (around 14%) of chromosomal disorders in structurally abnormal and growth retarded fetuses, karyotyping is discussed in most cases following the detection of most major and some minor anomalies or so-called soft markers (Table 2.6). This is particularly recommended when more than one abnormality or marker is seen, as the incidence of aneuploidy increases from less than 2% in fetuses with isolated defects to 29% in those with multiple anomalies.25
Fetal Doppler
Intrauterine growth restriction (IUGR) occurs in 5–10% of all pregnancies and increases the risk of hypoxemia and acidemia, possibly resulting in perinatal death or significant morbidity in adult life.26 Fetal weight can be estimated using a formula incorporating a combination of sonographic fetal measurements, usually HC, AC and femur length. A fetus with a weight below the 10th centile is defined as small for gestational age, but not necessarily growth restricted. The use of fetal and maternal Doppler has helped in making the prenatal distinction between constitutionally small fetuses, those that are small because of an underlying genetic etiology, and those where placental insufficiency (IUGR) is the cause. The interpretation of the hemodynamic changes in various fetal arterial and venous waveforms demonstrated by Doppler ultrasound make it possible to focus on the fetoplacental circulation and to evaluate the condition of the growth-restricted fetus.
The umbilical artery was the first vessel to be evaluated by Doppler velocimetry. In normal fetuses a progressive rise in the end-diastolic velocity and subsequent decrease in pulsatility index can be demonstrated with advancing gestation. In IUGR fetuses an increase in pulsatility index can occur. With progressive deterioration of the fetoplacental circulation the umbilical artery waveform will change, showing absent or reversed end-diastolic blood flow. Growth-restricted fetuses with abnormal umbilical waveforms often have a poorer perinatal outcome than those with normal waveforms.27
There is a direct correlation between umbilical artery Doppler and changes in the diastolic and mean blood flow velocity in the middle cerebral artery (MCA). During fetal hypoxia a pathophysiological adaptation occurs which results in preferential perfusion of vital organs. In the fetal brain this results in decrease in pulsatility index in the MCA as a direct consequence of vasodilation,28 also referred to as redistribution or the ‘brain-sparing effect’.
Besides the brain, the myocardium is also spared (the ‘heart-sparing effect’), leading to abnormal velocimetry of the aorta and inferior vena cava.29 Doppler velocimetry has been applied to a variety of other fetal arteries and veins. Recent studies provide evidence that increased pulsatility index in the ductus venosus seems to be closely related to the presence of metabolic acidemia.30,31 These studies furthermore provide evidence that IUGR fetuses develop changes in Doppler velocimetry in a time-dependent, progressive sequence. Increase in umbilical artery pulsatility index occurs first, followed by a decrease in MCA pulsatility index. When the fetus becomes further hypoxic this will lead to Doppler changes in ductus venosus and aortic flow. The clinical utility of these Doppler changes in optimal timing of delivery in IUGR fetuses remains to be established.
On the maternal side of the fetal circulation the flow in the uterine arteries is used as a screening tool, at 24 weeks of gestation, for placental insufficiency likely to result in pre-eclampsia or IUGR.32 Abnormal changes in the uterine artery waveform are characterized by increased impedance indices and ‘notches’ in the waveform. Patients displaying abnormal uterine artery waveforms should be seen at regular intervals for monitoring of maternal wellbeing and fetal growth.
Beside its usefulness in IUGR fetuses, assessment of Doppler changes in the MCA is becoming an accepted non-invasive method for diagnosis of fetal anemia in red blood cell alloimmunized pregnancies. Among fetuses at risk for immunological hydrops, 25% will need intrauterine transfusions. Up to 40% of the remaining group will develop mild to moderate anemia and hyperbilirubinemia postnatally, requiring phototherapy. In uncomplicated pregnancies the blood velocity in the cerebral vessels increases with advancing gestation. An increased MCA peak systolic velocity above 1.5 multiples of the median will identify 88% of those fetuses requiring intrauterine transfusion.33
ABNORMALITIES IN DIFFERENT SYSTEMS
Cranial and spinal abnormalities
Many intracranial abnormalities are detectable at the time of the routine 20-week scan. One of the most common findings is that of choroid plexus cysts (CPCs), which occur in 1–2% of all fetuses. These can be uni- or bilateral, single or multiple (Fig. 2.6). More than 95% resolve spontaneously before 26 weeks’ gestation. When seen with other soft markers, sonographic abnormalities or other risk factors for aneuploidy, such as maternal age ≥ 36 years or positive maternal serum screening, they may be associated with trisomy 18. They are not associated with any other adverse outcome. In the vast majority of cases CPCs are a benign transient finding with no clinical significance.34 Other major abnormalities such as holoprosencephaly, anencephaly and posterior fossa cysts (Fig. 2.7) may be detected at the time of a routine first or second trimester scan. However, unfortunately, many of the sonographic signs associated with other intracerebral anomalies develop later in pregnancy and are thus less amenable to detection in the second trimester. These include many, but not all, cases of hydrocephaly, microcephaly, agenesis of the corpus callosum, arachnoid cysts (Fig. 2.8) and tumors. Many intracerebral abnormalities will therefore only be detected if the scan is initiated because of a clinical indication later in pregnancy,10 whereas detection of anomalies such as anencephaly approaches 100% in the first35 and second trimesters (see Tables 2.2 and 2.3).
One of the most common serious congenital anomalies is open neural tube defect (Fig. 2.9). Open spina bifida can be identified by examination in all three planes (Fig. 2.5), sometimes just by loss of alignment of the vertebral bodies and absence of skin covering, but in more severe cases by identification of gross kyphoscoliosis. The diagnosis can be particularly difficult if the fetus is in the breech position, and low sacral lesions are readily overlooked. Identification of the cranial signs associated with spina bifida has resulted in a significant improvement in the diagnosis of this lesion, as the head is readily examined. In the presence of spina bifida the skull has a lemon shape in 98% of cases when scanned before 24 weeks, whilst the cerebellum is banana shaped in 72% (Fig. 2.10).36 These signs are also seen in 1% of normal fetuses.37 Ultrasonographic detection of open neural tube defects compares favorably with maternal serum alfa-fetoprotein (MSAFP) measurements, which can identify around 80% of fetuses with spina bifida.