Fig. 1.
The four most common strategies for bioconjugation to gold nanoparticles. The tear-shaped cartoon represents the biomolecule to be linked to the gold nanoparticle. Only the important functional groups for linking chemistry are shown, additionally the monolayer on the gold nanoparticle is also not depicted. (a) Direct bonding approach via ligand place exchange. (b) Sulfo-NHS–Ester Amine coupling approach. (c) Thiol–Maleimide coupling approach. (d) Ni–NTA Chelation approach.
1.1 Colloidal Gold: Immunogold
Colloidal gold has found use in EM imaging of biological tissues since at least 1971, when Faulk and Taylor demonstrated that by simply adsorbing antibodies on the surface of the particle they could be rendered immunoactive (17). Such commercially available immunogold probes have been successfully used in many electron microscopy experiments (18–21). Additionally, selective bio-targeting of immunogold derivatives can be observed by measuring the surface plasmon resonance (SPR) of conjugates (22).
Immunogold probes are typically prepared by direct physisorption of antibodies or proteins to the colloidal gold surface. The nanoparticles used for immunogold preparations are generally <30 nm in diameter; in some cases 1–3 nm colloidal gold has been prepared, however the samples are typically not monodisperse or stable over long periods of time (23). Generally, it is recommended to prepare protein adsorbed colloidal gold near the pI of the protein that will be used. Although labeling is more efficient at higher pH ranges, protein unfolding can occur at higher pH values, limiting biological function and thus utility in an EM experiment. Additionally, it is important to limit the amount of protein adsorbed to colloidal gold probes to prevent desorption, which allows for competition in binding to the target (24).
As seen above, the use of colloidal gold in EM of biological tissues generally takes advantage of noncovalent strategies. Aside from antibody adsorption, another favorable electrostatic interaction used to conjugate biomolecules to colloidal gold includes the avidin–biotin interaction. Copland et al. demonstrated the successful conjugation of a biotinylated monoclonal antibody (Mab), Herceptin® (Mab for HER2/neu receptor over-expressed in early stage breast cancers) (25), to 40 nm streptavidin-coated gold nanoparticles (26).
Colloidal gold conjugates can be purified through repeated centrifugation, where the particle conjugates are collected as a “pellet” and then resuspended in a buffer of choice following supernatant removal. Typically this will be repeated a few times as “wash steps” before final resuspension of the purified conjugate. Colloidal gold particles are generally not amenable to gel electrophoresis purification and visualization methods that are typically successful for smaller gold nanoparticles. Because immunogold is a commercially available material (Nanopartz, Nanoprobes, Ted Pella, BBI, Electron Microscopy Sciences), with well-established protocols available for their production, we do not give detailed protocols in this chapter for electrostatic conjugation to colloidal gold nanoparticles.
1.2 Triphenyl Phosphine Protected Clusters: Undecagold and Nanogold ®
Bioconjugation of gold nanoparticles, especially the commercially available Nanogold® (1.4 nm) and undecagold [Au11(P(C6H5)3)7, 0.8 nm] derivatives, has found widespread use in electron microscopy for biological imaging (27). Nanogold® in particular has been demonstrated to successfully penetrate 40 μm deep into tissue (28). The smaller size of these clusters in comparison to colloidal gold allows for enhanced stability because linking chemistry takes advantage of covalent bonding strategies. Therefore, the conjugates do not exhibit desorption over time as colloidal gold conjugates do (24). Additionally, unlike colloidal gold, undecagold and Nanogold® do not aggregate over time. These smaller probes are usually visible through TEM alone in very thin specimens; however, silver enhancement can be utilized for increased visibility if necessary (29). Silver enhancement of these probes also allows them to be visible using bright field microscopy (17) as well as Scanning Electron Microscopy (30).
Nanogold® has emerged as a more popular choice than undecagold for biolabeling EM markers presumably due to its larger size, making it visible in unprocessed micrographs. Additionally, it possesses greater stability in the electron beam than undecagold. Nanogold® is sold in two reactive forms, baring either a monomaleimide or monosulfo-NHS ester functionalities which allow conjugation to antibodies via cysteine residues, and lysine residues and terminal amines respectively (see Fig. 1b, c). These reactive strategies have been extended to multiple other biomolecules including oligonucleotides (31), peptides (32), carbohydrates (33), etc. Additionally, Nanogold® conjugates can be prepared by taking advantage of a common protein tag—polyhistadine. Most proteins are engineered to contain His6 tags as a purification handle when bound to a Ni(II)–NTA (nitriloacetic acid) column. This chemistry can also be applied to Nanogold® by coupling His6 tagged proteins with commercially available particles modified to contain Ni(II)-NTA on the surface (see Fig. 1d). Additionally, Kogot et al. demonstrated that 1.5 nm triphenylphosphine capped nanoclusters were capable of conjugation to His6 without the use of Ni-NTA modified particles (34).
Nanogold® conjugates are separated readily from unreacted starting materials using gel filtration, gel electrophoresis, ion-exchange chromatography or high performance liquid chromatography. Because Nanogold® is a commercially available material (Nanoprobes.com, Yaphank, NY), with available protocols once purchased, detailed instructions for labeling will not be provided in this chapter.
1.3 Thiolate Monolayer Protected Clusters
Because we are most experienced working with thiolate monolayer protected gold clusters (MPCs), the rest of this chapter and protocols concern labeling chemistries of thiolate MPCs. MPCs ranging from 1 to 3 nm in diameter fit into what is considered a “magic number” regime, where particles have filled superatomic electron orbitals (35), yielding enhanced particle stability (analogous to the stability noble gases exhibit from a filled outermost orbital of electrons, e.g., the octet rule). These MPCs are composed of a crystalline gold core, where the outermost gold atoms are protected by bridging organothiolate ligands and are of the general formula Au n (SR) m . Magic number MPCs exist in molecularly defined sizes (general formulae: Au25(SR)18, Au38(SR)22, Au68(SR)34, Au102(SR)44, Au144(SR)60) and in some cases crystallographically defined sizes (Au25(SR)18, Au38(SR)22, Au102(SR)44) (36–38). In some cases, synthetic attempts will yield multiple cluster core sizes within the magic number regime, which can be separated readily from each other by fractional precipitation methods (see Subheading 3.2) or liquid chromatography.
The nanoclusters we work with preferentially (Au25, Au102, Au144) fit into the magic number regime of molecule-like clusters. Both Au102(pMBA)44 and Au144(pMBA)60 are protected by pMBA (para-mercaptobenzoic acid) ligands, which provide the clusters with both water solubility as well as a reactive terminal functional group (COOH). The carboxylic acid ligands on Au102(pMBA)44 and Au144(pMBA)60 allow for NHS–EDC amide bond coupling with any commercially available terminal amines and proteins that display lysine residues, in addition to direct bonding by the place exchange reaction with thiolated ligands and proteins displaying cysteine residues. We have had success labeling Au144(pMBA)44 with IgG using both of the aforementioned strategies, depicted in Fig. 2. Detailed protocols for each of these strategies are discussed in Subheadings 3.4 and 3.5 along with helpful tips that should be considered when preparing any bioconjugate.
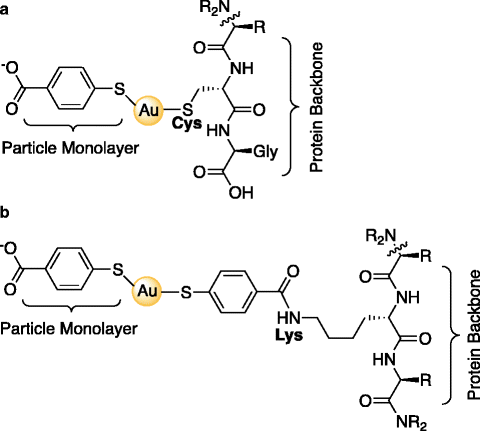

Fig. 2.
Schematic of protein conjugation strategies presented in the Subheading 3: IgG@Au144(pMBA)60. (a) Direct bonding via ligand place exchange between a near terminal Cysteine residue and the Au MPC. (b) NHS–EDC coupling between a carboxylic acid of the pMBA ligand layer and a primary amine on a Lysine residue within the protein. Note that for clarity, only one intact pMBA of the ligand layer (total 60 ligands) is represented in these depictions.
Water-soluble gold clusters are amenable to functionalization with organic thiolates and proteins exposing cysteine residues via ligand place exchange (13). The ligand place exchange reaction allows for a stoichiometric exchange of incoming thiolate molecules for native thiolate ligands that protect the gold cluster. For example: Au144(pMBA)60 + 4 SR ® Au144(pMBA)56(SR)4 + 4 pMBA. The ligand place exchange reaction is employed for nearly all applications of gold particles and has been studied in both kinetic and mechanistic detail since its discovery. The rate and extent of reaction depend on the nature of the incoming and outgoing ligands as well as the oxidation state of the cluster core (13, 14). It has been demonstrated that incoming ligands possessing electron-withdrawing character increase the rate of the reaction. Additionally, oxidatively charging the cluster core increases the reaction rate as well. Any incoming thiolate molecule can be used for this reaction including small molecule organothiolates, proteins displaying cysteine residues, as well as oligonucleotides modified with a 5′ or 3′ thiol. Gold conjugates have been prepared using this strategy for a variety of biomolecules including antibodies (39), proteins (40–43), peptides (44, 45), oligonucleotides (46) and small biologically active molecules (4).
Depending on the functionality of the terminal pendant group on the thiolate ligand layer protecting the gold cluster, various chemical opportunities arise to complete a conjugation or labeling reaction. The MPCs we work with display carboxylic acids, which allow for reactivity with primary amines via NHS–EDC coupling. Similarly, MPCs displaying primary amines can theoretically undergo the same reaction with commercially available carboxylic acids or esters. Nearly any functionalized thiolate can undergo place exchange with MPCs providing additional reactive possibilities. The breadth of standard organic chemistry reactions that could be completed on appropriately functionalized MPCs with variously functionalized small molecules has many possibilities that have not even been discovered yet.
2 Materials
2.1 Reagents
1.
Hydrogen tetrachloroaurate.
2.
Para-mercaptobenzoic acid (must be sourced from TCI America).
3.
Nanopure H2O.
4.
Methanol.
5.
Ammonium Acetate.
6.
Sodium Hydroxide.
7.
Sodium Borohydride.
8.
N-Hydroxysuccinimide (NHS).
9.
1-Ethyl-3-(3-dimethylaminopropyl) carbodiimide (EDC).
10.
Tris(2-carboxyethyl)phosphine (TCEP).
11.
Thiolated biomolecule for labeling (IgG is used in this protocol).
12.
Aminated biomolecule for labeling (IgG is used in this protocol).
13.
50 mM Phosphate Buffer, pH 7.5.
14.
0.5 M Phosphate Buffer, pH 7.5.
15.
10× Tris–Boro–EDTA (TBE): 890 mM Tris base, 890 mM Boric acid, 20 mM EDTA, pH 8.3.
16.
1× TBE: 89 mM Tris base, 89 mM Boric acid, 2 mM EDTA, pH 8.3.
17.
2× Nonreducing Loading Dye: 10 mL H2O, 5 mL glycerol, 4 mL 1 M Tris–HCl pH 6.8, 1 g SDS, 0.6 mL 1% bromophenol blue solution (see Note 1).
18.
Acrylamide (40%, 19:1).
19.
10× Tris–Glycine SDS Running Buffer: 250 mM Tris, 1.92 M glycine, 1% SDS, pH 8.3.
20.
1× Tris–Glycine SDS Running Buffer: 25 mM Tris, 192 mM glycine, 0.1% SDS, pH 8.3.
21.
Coomassie Staining Solution: 40% methanol, 10% acetic acid, 50% H2O, 0.1% (w/v) Coomassie Brilliant Blue-R250.
22.
Destaining Solution: 40% methanol, 10% acetic acid, 50% H2O.
23.
Au102(pMBA)44 or Au144(pMBA)60.
2.2 Equipment
1.
Lyophilizer.
2.
Centrifuge(s) capable of spinning 50 mL conicals and 1.5 mL Eppendorf® tubes.
3.
Vortexer.
4.
Polyacrylamide Gel Electrophoresis Box.
5.
Power Supply (at least 200 V).
6.
White Light Box.
7.
Scalpel.
8.
Chromatography Equipment.
9.
1.5 mL Eppendorf® tubes.
10.
50 mL conicals.
11.
Rocker or shaking rotator.
3 Methods
Herein, we have included a protocol for the preparation of the negatively charged MPC Au144(pMBA)60 (see Note 2), as well as two conjugation strategies: direct bonding via ligand place exchange and NHS–EDC coupling. Isolation, purification and characterization methods are also included.
3.1 Synthesis of Au144(pMBA)60 MPC
1.
Prepare 95.2 mM pMBA in 0.3 M NaOH. Allow stirring overnight (see Note 1).
2.
The following day, prepare 28 mM HAuCl4·3H2O in water.
3.
The Au(I)-pMBA polymer is then generated in a methanol: water solvent (47% methanol, 53% water) with a 3:1 molar excess of pMBA (0.432 mmol, 9 mM final volume) in relation to HAuCl4 (0.144 mmol, 3 mM final volume). This can be prepared in a 50 mL conical by combining in the following order (see Note 3): methanol, nanopure H2O, HAuCl4 solution, and finally pMBA solution. We have had success preparing this polymer on a scale of 48 mL final volume (see Note 4). The polymer is then allowed to stir overnight prior to reduction.
4.
The following day, prepare a 150 mM solution of NaBH4 in nanopure H2O (see Note 5) and immediately add 0.5 molar equivalents (relative to Au) to the polymer. We have had success preparing Au144(pMBA)60 at volume scales between 3 and 48 mL (see Note 4). The reduction is allowed to proceed at room temperature with vigorous stirring or vortexing for a maximum of 2 h (see Note 6).
5.
The product is then precipitated with the addition of ammonium acetate (80 mM final volume) and methanol (equivalent volume to reaction scale volume). Centrifugation at 3220 rcf for 10 min at 4°C will be sufficient to “pellet” the MPC out of solution. The supernatant is then decanted, the MPCs are resuspended in nanopure H2O and then precipitated again as described above to remove any remaining residual thiolate or NaBH4 impurities.
6.
The product is then resuspended in nanopure H2O. For a 25 mL scale, 0.5 mL is an appropriate volume to add.
7.
Product purity is quickly assessed by polyacrylamide gel electrophoresis (PAGE). Run the MPC product on a 15% polyacrylamide gel (19:1, 5% glycerol final concentration) in 1× TBE at 110 V for 1–1.5 h. The sample can be loaded into the gel by combining 5 μL of 50% glycerol with 1 μL of MPC sample. If the gel illustrates a large abundance of one band, but a smear reaching towards the well, a fractional precipitation will be required to remove larger material. This protocol is described in Subheading 3.2.
3.2 Fractional Precipitation Protocol: Purification of Au144(pMBA)60 MPC
Typically, the synthesis described above will generate an abundance of Au144(pMBA)60; however, sometimes larger MPC impurities will also be generated, requiring their removal from the desired cluster core size. This is accomplished using a fractional precipitation method, where increasing amounts of precipitating agent are added to the MPC suspension to remove larger products from the desired product: Au144(pMBA)60. This is an effective purification method because larger MPCs require less precipitant (in this case methanol) to crash out of solution than smaller MPCs (likewise smaller MPCs require more precipitant). If fractional precipitation purification is necessary, the following steps should be taken.
1.
The MPCs in need of purification should be suspended in approximately 9 mL of nanopure H2O in a 50 mL conical.
2.
Add ammonium acetate to a final concentration of 80 mM.
3.
Add methanol to a final concentration of 20%.
4.
Centrifuge the sample at 4,000 rpm for 10 min at 4°C. This should remove the majority of really large products if they are present.
5.
Decant the supernatant into a fresh 50 mL conical. Save pellet to check by PAGE.
6.
Add methanol to the supernatant to a final concentration of 40%.
7.
Centrifuge the sample again at 4,000 rpm for 10 min at 4°C. This should remove products that are slightly larger than Au144(pMBA)60 if they are present.
8.
Decant the supernatant into a fresh 50 mL conical. Save pellet to check by PAGE.
9.




Add methanol to a final concentration of 60%.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree