▪
Mediastinal Lesions
Although it may not be difficult to identify a mediastinal mass on cross-sectional imaging, it can be challenging to determine its nature. Diagnostic specificity is critical to prevent unnecessary intervention and its associated morbidity and expenditure. The aim of this chapter is to serve as a guide toward maximizing the benefit of current diagnostic imaging tools for mediastinal lesions to improve diagnostic accuracy and clinical management in the interests of patient care. Emphases will be placed on anterior mediastinal lesions, which have historically been a source of confusion, and on thoracic MRI, which has heretofore been underutilized.
Current primary diagnostic imaging tools for the mediastinum include chest radiography (CXR), contrast esophagography, chest computed tomography (CT), 18-fluorodeoxyglucose positron emission tomography ( 18 FDG-PET), and thoracic magnetic resonance imaging (MRI). These diagnostic imaging tools can occasionally supplant endoscopic evaluation, surgical exploration, and biopsy when used to their full potential.
Mediastinal lesions are often detected incidentally on chest imaging performed for an unrelated reason. Alternatively, patient symptoms or signs can lead to a clinical suspicion of a mediastinal mass, whether due to gradual enlargement or rapid lesion enlargement because of hemorrhage or lesion rupture, the occurrence of which may lead to airway, vascular, esophageal, or nerve root compression. Presenting symptoms and signs may include shortness of breath, dysphagia, chest pain, hoarseness (from recurrent laryngeal nerve involvement), hemidiaphragmatic paralysis (from phrenic nerve involvement), and, rarely, a radiculopathy.
Designating the mediastinal compartment from which a mass arises is the first step toward narrowing the differential diagnosis because it leads to consideration of the structures that dwell in that particular space and their cellular composition. Both the center method and structure displacement method can be helpful for this analysis. The center method presumes that the center of the largest part of the lesion on an axial image designates the compartment of origin. The structure displacement method is based on the premise that a large lesion typically displaces anatomic structures from other compartments adjacent to its own.
The second step toward narrowing the differential diagnosis is tissue characterization. Overall, CXR does this least well, CT is better, and MRI is best to characterize tissue.
The third and last step is consideration of statistical probability and clinical context. Therefore the questions to ask when presented with a mediastinal lesion are as follows:
- 1.
Which compartment?
- 2.
What tissue type?
- 3.
What is the most likely lesion, both statistically and contextually?
The recently proposed tricompartmental definition of mediastinal compartments by Carter et al. and the International Thymic Malignancy Interest Group published in the Journal of Thoracic Oncology will be used for the purposes of lesion compartmentalization in this chapter. This updated definition of mediastinal compartments is based on their delineation by cross-sectional imaging, rather than by CXR. All three mediastinal compartments are described to extend from the thoracic outlet to the diaphragm. The anterior mediastinal, or prevascular, compartment is defined as all structures posterior to the sternum and anterior to the pericardium. The middle mediastinum, or visceral compartment, extends from the anterior margin of pericardium posteriorly to a vertical line traversing the junction between the anterior and middle thirds of the thoracic vertebrae. The posterior mediastinum, or paravertebral compartment, contains all mediastinal structures posterior to the above-mentioned vertical line and is contained by the inner margin of the chest wall, extending posterolaterally to the lateral margin of thoracic spinal transverse processes.
▪
Problem Solving With Imaging
Chest Radiography
On CXR, the first indication of a mediastinal mass may be the shift, deformation, or silhouetting of mediastinal anatomy, contours, or lines or, alternatively, the obliteration of spaces typically filled with air, such as the retrosternal clear space. These mediastinal lines have been discussed earlier in Chapter 2 . For example, widening or thickening of the anterior junction line may be an early indication of an anterior mediastinal or prevascular mass on a frontal CXR. Anterior mediastinal masses may also be detected when soft tissue attenuation replaces the normally air-filled retrosternal clear space. Middle mediastinal or visceral compartmental masses may be detected by silhouetting of the paratracheal stripes, fill-in of the aortopulmonary window, or distortion of the azygoesophageal recess. Posterior or paravertebral compartmental mediastinal masses may be deduced if the mass silhouettes the paraspinal lines or vertebrae.
Although the CXR may suggest a mediastinal mass, it seldom can achieve diagnostic specificity because of the lack of soft tissue contrast between mediastinal structures on CXR. Therefore, once a mediastinal mass is suggested by CXR, cross-sectional imaging is usually the next step, whether by chest CT or MRI.
Contrast Esophagography
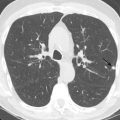
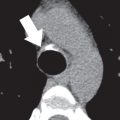
Contrast esophagography may depict a mediastinal mass directly or incidentally infer the presence of a mediastinal mass by outlining an intraluminal mass, by highlighting an aberrant course of the esophagus, or by demonstrating mass effect on the esophagus by an adjacent mass ( Fig. 34.1 ).



Contrast esophagography can be quite helpful when there is concern for esophageal fistulae and perforation. It is performed most safely with a low-osmolar, iodinated contrast agent such as iopamidol, with administration of barium sulfate reserved for cases in which the initial low-osmolar contrast agent esophagram is negative. CT and MRI may also depict these findings ( Figs. 34.2 and 34.3 ).







Chest CT
Chest CT not only more readily depicts the mediastinal compartment in which the lesion resides, but also provides some insight into the nature of the tissue, including better delineation of lesion morphology than CXR, some indication of the lesion’s cystic or solid nature and enhancement, heterogeneity versus homogeneity of attenuation, the presence or absence of gross fat and calcification, and lesion invasiveness.
Dual-energy CT with IV contrast may offer some advantage over standard, single-energy CT in differentiating cystic from solid lesions because of the material decomposition images it provides. Region of interest (ROI) assessment of attenuation is not absolute with this form of CT, however. These images are generated by the subtraction of two basis materials (e.g., water, calcium, iodine). The water images, also known as virtual noncontrast images , are generated by digitally subtracting iodine (IV contrast) from the image, aiding discrimination of enhancing from nonenhancing lesions and allowing quantification of water content in mg/mL. The water images also facilitate the differentiation of calcium from iodine on postcontrast chest CT. The iodine images can also help differentiate high-attenuation hemorrhage and other nonenhancing tissues from enhancing tissue. Dual-energy CT enables lower IV contrast doses because its images can be reconstructed at several different virtual monoenergetic levels, ranging from 40 to 190 keV, with the lower keV images yielding better iodine contrast and the higher keV images exhibiting diminished noise and artifacts.
FDG-PET
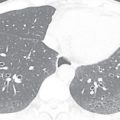
Beyond its seminal role in cancer staging, 18 FDG-PET imaging has a limited role in the evaluation of mediastinal lesions. Its strength is its ability to demonstrate the level of metabolic activity within a lesion and to ferret out clinically significant lesions that are difficult to detect by CT, either because they are very small but metabolically active or because of their isoattenuation with adjacent tissue. Many lesions may be too small to detect with 18 FDG-PET imaging, despite their malignant nature, if they are insufficiently metabolically active. In addition, many benign mediastinal lesions, including thymic hyperplasia and inflammatory mediastinal cysts, demonstrate 18 FDG-PET avidity ( Fig. 34.4 ) and can therefore be misinterpreted as malignant or, at least, meriting resection to exclude malignancy. Even a normal thymus may be 18 FDG-PET–avid. In cases of indeterminate mediastinal lesions on CT, it is often valuable to perform MRI prior to consideration of PET to ascertain if there are lesion features favoring a benign diagnosis, instead of referring the patient directly for 18 FDG-PET imaging. MRI in select cases has the potential to spare the patient morbidity and radiation exposure and our health care system the cost of unnecessary surgery. PET is substantially more expensive and time-consuming than MRI and exposes the patient to a significant dose of radiation (effective dose range of 8–26 mSv), unlike MRI.




Thoracic MRI
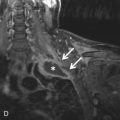
The superior tissue contrast of MRI and resultant superior tissue characterization, when compared with CT, enables the following: (1) definitive discernment between cystic and solid lesions ( Fig. 34.5 ); (2) demonstration of the simplicity or complexity of a cystic lesion, (3) detection of blood products ( Figs. 34.6 and 34.7 ); (4) discernment of the presence of smooth muscle and collagenous and fibrous material based on relative or actual T2 hypointensity ( Figs. 34.8 and 34.9 ; see Fig. 34.3 ); (5) delineation of the enhancement pattern of a lesion by dynamic contrast enhancement (DCE; Fig. 34.10 ); and (6) demonstration of the degree of restricted diffusion with apparent diffusion coefficient (ADC) mapping (see Fig. 34.8 ), all without any radiation exposure. The high soft issue contrast of MRI may demonstrate chest wall and neurovascular invasion better. Free breathing steady-state free precession (SSFP) MRI and cardiac tagging by MRI can further assist in the assessment of mediastinal mass invasion of adjacent structures. MRI may also demonstrate the compartment in which a lesion resides better, changing the differential diagnosis. For example, it can be difficult to discern whether a lesion is mediastinal or paramediastinal, not only by CXR, but also by CT. In such cases, the higher soft tissue contrast and superior definition of soft tissue planes by MRI can be helpful ( Fig. 34.11 ). MRI does not readily demonstrate calcifications, however. When present, calcifications on MR are usually T2-hypointense. These capabilities of MRI often lead to greater diagnostic specificity in the evaluation of mediastinal lesions and may provide additional valuable information for clinical management, whether medical or surgical.

































Because of its lack of ionizing radiation, MRI may be used to screen for or follow up lymphadenopathy in young, pregnant, and/or radiation-averse patients with lymphoma and chronic lymphocytic leukemia, melanoma metastases in pregnant patients (without IV gadolinium administration), endometriomas, paragangliomas and teratomas in patients with predisposing genetic mutations (e.g., a mutation in the succinate dehydrogenase complex, subunit D integral membrane protein [SDHD] gene), and teratomas in patients with anti– N -methyl- d -aspartate receptor antibodies, respectively. For sample mediastinal and thymic MRI protocols, see Table 34.1 and Box 34.1 .
| PULSE SEQUENCE a | MANUFACTURER | ||
|---|---|---|---|
| GE | SIEMENS | PHILIPS | |
| BH axial and sagittal SSFP balanced GRE | FIESTA | True FISP | BFFE |
| BH coronal ultrafast spin-echo T2 | SSFSE | HASTE | UFSE |
| BH sagittal ultrafast spin-echo fat-saturated T2 | SSFSE | HASTE | UFSE |
| BH axial in- and out-of-phase T1 (chemical shift) ultrafast GRE | FSPGR | Turbo FLASH | TFE |
| BH cardiac-gated double IR T2 | Double IR FSE T2 | Double IR TSE T2 | Double IR UFSE T2 |
| BH pre- and post–three-dimensional ultrafast GRE (with postgadolinium imaging) acquired at 20 s (axial), 1 min (axial), 3 min (sagittal), and 5 min (axial) b | LAVA | VIBE | THRIVE |
| Optional coronal/sagittal BH STIR | Fast STIR | Turbo STIR | STIR TSE |
| Optional diffusion-weighted echoplanar imaging with b values of 50, 400, 800 | eDWI | DWI | DWI |
| Optional BH cardiac-gated double IR | Double IR prep FSE T1 | Double IR prep TSE T1 | Double IR prep UFSE T1 |
| Optional BH cardiac-gated sagittal double IR fat-saturated T2 | Double IR FSE fat-saturated T2 | Double IR TSE fat-saturated T2 | Double IR UFSE fat-saturated T2 |
| Optional respiratory-triggered radially acquired FSE T2 (periodically rotated overlapping parallel lines with enhanced reconstruction) | PROPELLER | BLADE | MultiVane |
| Optional respiratory-triggered radially acquired FSE fat-saturated T2 (periodically rotated overlapping parallel lines with enhanced reconstruction) | PROPELLER | BLADE | MultiVane |
| Optional respiratory-triggered axial-driven equilibrium T2 | FRFSE | RESTORE | DRIVE |
| Optional respiratory-triggered axial-driven equilibrium fat-saturated T2 | FRFSE | RESTORE | DRIVE |
a Default slice thickness is 4 mm for all pulse sequences.
b Option to add, for example, postgadolinium axial imaging 2 and 4 minutes after injection. Imaging planes can be changed to optimize lesion characterization.
Breath-hold (BH) axial steady-state free precession imaging
BH axial in- and out-of-phase ultrafast gradient echo (GRE) T1
BH, electrocardiographic-gated axial double inversion recovery T2
If needed, b
b If the thymic lesion uniformly suppresses on opposed-phase imaging, with a chemical shift ratio ≤0.7 or signal intensity index >10%, no IV contrast administration is needed.
BH pre- and post–three-dimensional ultrafast GRE with automated subtraction (postgadolinium imaging) acquired at 20 seconds (axial), 1 minute (axial), 3 minutes (sagittal), and 5 minutes (axial)
▪
Mediastinal Lesions by Compartment
Table 34.2 provides a list of mediastinal lesions categorized by compartment, whether prevascular, visceral, or paravertebral.
| ANTERIOR MEDIASTINUM, PREVASCULAR COMPARTMENT a | MIDDLE MEDIASTINUM, VISCERAL COMPARTMENT | POSTERIOR MEDIASTINUM, PARAVERTEBRAL COMPARTMENT |
|---|---|---|
| Lymphadenopathy | Lymphadenopathy | Lymphadenopathy |
| Thymic lesions (cyst, hyperplasia, neoplasms) | ||
| Thyroid and parathyroid lesions | Thyroid lesions | Neurogenic tumors including neurofibroma, schwannoma, paraganglioma Lateral meningocele |
| Ascending aortic aneurysm Aortic arch aneurysm Dilated main pulmonary artery Aberrant right or left subclavian artery | ||
| Tracheal lesions | ||
| Esophageal lesions | ||
| Paraganglioma and other neurogenic tumors | ||
| Descending aortic aneurysm | ||
| Germ cell tumors | Pancreatic pseudocyst | Pancreatic pseudocyst |
| Pericardial or mesothelial b cysts | Mesothelial cysts | Mesothelial cysts |
| Extramedullary hematopoiesis | ||
| Morgagni hernia | Hiatal hernia | Bochdalek hernia |
| Bronchogenic cysts (rarely) | Foregut duplication cysts | Foregut duplication cysts |
| Abscess | Abscess | Abscess |
| Hematoma | Hematoma | Hematoma |
| Fibrosing mediastinitis | Fibrosing mediastinitis | Fibrosing mediastinitis |
| Hemangioma | Hemangioma | Hemangioma |
| Lymphangioma | Lymphangioma | Lymphangioma |
| Sarcoma | Sarcoma | Sarcoma |
a Based on the new International Thymic Malignancy Interest Group classification scheme of mediastinal compartments referenced at the end of this chapter as Carter et al. A modern definition of mediastinal compartments. J Thorac Oncol. 2014;9(9):S97–S101.
b Mesothelial cysts can theoretically be found anywhere in the body where mesothelium exists.
Prevascular or Anterior Mediastinal Compartment
Normal anatomic structures in the prevascular compartment of the mediastinum include the thymus, lymphatics, lymph nodes, vessels, and fat. Therefore lymphadenopathy, whether from an inflammatory, metastatic, or lymphoproliferative cause, and thymic lesions comprise the bulk of lesions in this compartment. Thymic lesions include benign nonneoplastic lesions such as thymic hyperplasia and thymic cysts, benign neoplastic lesions such as a mature teratoma and thymolipoma, and malignant neoplastic lesions, the most common of which is a thymoma. More rare thymic neoplasms include thymic carcinoma and thymic neuroendocrine tumor (formerly referred to as thymic carcinoid). Germ cell tumors are less common than most of the above-mentioned thymic lesions, with mature teratoma representing the most common germ cell tumor. Lymphangiomas and hemangiomas may also reside in the prevascular compartment in adults, although they are rare lesions.
Normal Anterior Mediastinal Structures
Normal anterior mediastinal structures are sometimes misinterpreted as abnormal, and vice versa.
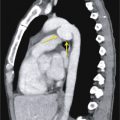
Pericardial Fat Pad Versus Pericardial Cyst
On CXR, when it is difficult to ascertain whether an apparent cardiophrenic angle mass represents a pericardial fat pad or pericardial cyst, it is reasonable to perform mediastinal MRI to avert radiation exposure and answer the clinical question definitively ( Fig. 34.12 ). Chest CT may alternatively be performed. Pericardial cysts may change location and morphology between examinations and are typically of water attenuation on CT and T1-hypointense and T2-hyperintense on MRI. Their CT attenuation and MR T1-weighted signal may be increased in rare cases of intralesional hemorrhage. Typically there is no appreciable wall enhancement of pericardial (mesothelial) cysts on CT or MRI.