, Foster D. Lasley2, Indra J. Das2, Marc S. Mendonca2 and Joseph R. Dynlacht2
(1)
Department of Radiation Oncology, CHRISTUS St. Patrick Regional Cancer Center, Lake Charles, LA, USA
(2)
Department of Radiation Oncology, Indiana University School of Medicine, Indianapolis, IN, USA
Definitions
A = Amplitude of a wave
ν = Frequency of a wave
λ = Wavelength of a wave
p = Momentum of a particle
h = Planck’s constant = 6.62 × 10−34 J s
c = Speed of light = 3 × 108 m/s
Bremsstrahlung: “braking radiation”; photons that are produced whenever a fast moving electron slows down near nucleus.
kilovolts peak (kVp): Maximum accelerating voltage of an X-ray tube.
Particulate Radiation
Particles include electrons, protons, carbon ions (and other ions), pions and whatever else comes out of big accelerators like CERN.
Photons and other bosons are NOT considered particles for the purpose of radiation therapy as they are considered carriers for forces – in the case of photons, electromagnetic force.
Relativistic energy equation – all particles must obey the law E = mc2, therefore as you increase the energy for particles already at 99.9 % of the speed of light, the mass increases (note that the particles get heavier but not larger in size).
The resting mass of a particle can be converted to pure energy if you destroy it.
Electromagnetic (EM) Radiation
Carried by photons (a type of boson).
Wave-particle duality – EM radiation can be either a wave or a particle (photons) depending on how you look at it. Actually, everything can be described as both, but photons are extra special because they carry electromagnetic force in the form of two sine waves (electric field and magnetic field) that are perpendicular to each other (Fig. 3.1).
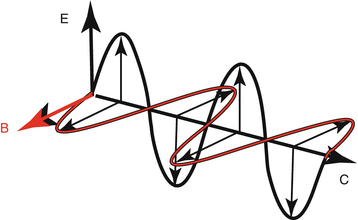
Fig. 3.1
Electromagnetic radiation wave form: there is an electrical sine wave (Black “E”) and a magnetic sine wave (Red “B”) that are perpendicular to each other.
Wave Equations
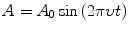
(3.1)
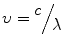
(3.2)
This means that you can always interchange ν and λ by dividing the speed of light (c) by the other term.
Photons possess momentum by p = h ν/c where c is the speed of light, h is planck’s constant and ν is frequency (we never use this).
Photons possess energy by E = h ν (we use this a little more often).
Since h and c are constants, we can rearrange and simplify everything to say electromagnetic wave length and energy are connected as:
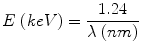
(3.3)
Electromagnetic Spectrum (Remember This from Fourth Grade?)
In order of increasing energy: Radio waves → microwaves → infrared → rainbow colors, light → UV rays → x-rays, gamma rays and Cosmic rays.
Here are a couple valuable points:
EM radiation actually becomes ionizing in the extreme UV spectrum since the ionization threshold for a hydrogen atom is 13.6 eV and higher (91.2 nm wavelength and smaller).
X-rays are >124 eV (around 10 nm) and therefore well above the energy threshold to cause ionizations.
By definition, X–rays come from electron interactions, γ–rays come from the nucleus (like the difference between electrons and beta particles).
They can have the same energies but are named based on their origin.
As a side-note, UV radiation can still cause chemical reactions by exciting valence electrons, altering chemical bonds without actually ionizing. This is why sun-tanning is bad and still cancer-causing even though there is no “ionizing” radiation involved.
Production of Radiation
Radioactive decay – for full details, see Chapt. 2.
Photons can be produced by the nucleus (γ–rays, ex: 60 Co) or by interactions of electron orbitals (X–rays, Ex: 125 I).
Electrons may be ejected as Auger electrons or as Beta Particles.
Alpha particles are produced by radioactive decay of heavy nuclei.
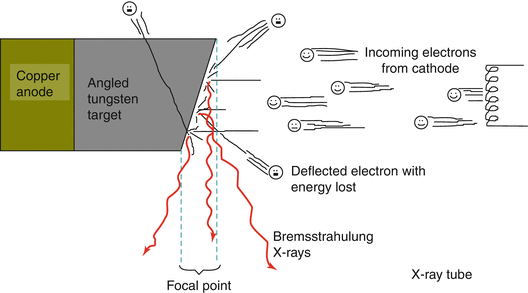
X–ray Tube
Diagnostic Energies
Used for plain X-ray imaging, mammography, CT scans, etc.
Brehmsstrahlung x–rays are produced whenever fast-moving electrons interact with matter.
An X–ray tube must first accelerate the electrons, in order to make X-rays through Bremsstrahlung.
Electrons are produced:
A Cathode (usually a tungsten wire) is heated, liberating electrons via thermionic emissions. These electrons float around the filament in a sort of cloud.
Electrons are Accelerated:
The slow moving cloud of electrons must be accelerated by a very high voltage electric field in a vacuum (must be a vacuum or else the electrons will collide with molecules).
Acceleration potential is measured in kilovolts (kV).
Electrons travel from the cathode to the anode, accelerating to between 50 and 99 % of the speed of light!
Electrons are Decelerated:
Get Clinical Tree app for offline access
The fast moving electrons run into a TARGET that is in front of the anode. The electrons have their paths bent and slowed down by high–Z material (tungsten or molybdenum) which produces Bremsstrahlung radiation (Fig. 3.2).