(1)
CyberKnife Centers of San Diego, Genesis Healthcare Partners, San Diego, CA, USA
Abstract
Stereotactic Body Radiotherapy (SBRT) describes a contemporary external beam radiotherapy method that utilizes hypofractionation, delivering a small number of large doses per fraction, potentially exploiting a radiobiologic advantage unique to prostate cancer, whose cells are thought to have a high sensitivity to fraction size. Although prostate cancer may have a greater sensitivity to fraction size than surrounding normal tissues such as rectum and bladder, translating to a theoretical therapeutic ratio improvement by SBRT hypofractionation, these surrounding normal tissues also have a relatively high sensitivity to fraction size, meaning they too are potentially damaged by large dose per fraction radiotherapy treatment. As such, tight margins and extreme accuracy are also a necessary prerequisite when delivering hypofractionated radiation treatment. Several very precise radiotherapy delivery technologies have emerged that satisfy this technical requirement, including both image-guided noncoplanar and image-guided or electromagnetic-guided gantry based systems. Considering the relatively narrow margins and high purported central biologic potency of SBRT, there are three different potential uses of this modality against prostate cancer:
SBRT used as monotherapy for clinically localized lesions that have a minimal risk of tumor extension beyond the immediate peri-prostatic region
SBRT used as a prostate boost, in conjunction with wider field “conventional” pelvic radiotherapy, against high-risk lesions that have a substantially greater risk of tumor extension beyond the immediate peri-prostatic region (i.e.—significant risk of positive seminal vesicles, lymph nodes)
SBRT used as a narrow margin salvage method used against recurrent prostate cancer—either following failed prior prostate radiotherapy (local retreatment) or against limited metastatic foci.
This chapter critically examines the use of SBRT applied to all three of the above-described prostate cancer scenarios.
Keywords
ProstateSBRTHypofractionationHDRBrachytherapyRadiosurgeryStereotacticRadiotherapyProstate Cancer11.1 Background and Rationale for Prostate SBRT
Prostate cancer is the most common visceral malignancy of men in the United States, accounting for 33 % of non-skin cases, with a total incidence greater than 200,000 cases/year [1]. The most important risk factor is age, with a median onset at age 68 years and a sharply rising incidence with increasing age beyond that [2]. There is significant heterogeneity in the incidence of this disease amongst different nations worldwide, with the greatest incidence of prostate cancer in Scandinavia and the lowest incidence occurring in Asia [1]. Dietary and perhaps other environmental factors likely play a role in the development of this disease, with the strongest evidence being the observation that over time, Asian men that reside in the United States develop a higher incidence of prostate cancer than their counterparts who remain in Japan or China [3]. Another important risk factor is family history, with greater than double the incidence of prostate cancer seen in men with a positive first degree relative family history of the disease [4]. Although prostate cancer remains the second leading cause of cancer-specific mortality among American males on an absolute basis, the prostate cancer death rates in the United States have been steadily decreasing since the advent of the “PSA era” in the early 1990s, likely due to a combination of earlier diagnosis by PSA screening as well as the widespread use of more effective surgical and radiotherapeutic local treatments.
11.2 Hypofractionation
Stereotactic Body Radiotherapy (SBRT) describes a contemporary external beam radiotherapy approach that utilizes hypofractionation, delivering a small number of large doses per fraction, potentially exploiting a radiobiologic advantage unique to prostate cancer, whose cells are thought to have a very low alpha-beta ratio (<2 Gy) that indicates a high sensitivity to fraction size [5–8]. The concept of hypofractionation is not a new idea at all, and such regimens were employed at the Christie Hospital (Manchester, UK) in the treatment of localized prostate cancer decades ago; this was most famously illustrated by the case of Sir Lawrence Olivier, who was successfully treated by a 36 Gy in 6 fraction regimen over 3 weeks (given as a 10 × 10 cm four field brick arrangement) in the late 1960s at St Thomas‘ Hospital, London, surviving apparently disease-free and complication-free for greater than 20 years thereafter [9].
Brenner and Hall evaluated the published efficacy outcomes of external beam and interstitial prostate brachytherapy in 1999 to derive an estimated α/β [alpha/beta] ratio of 1.5 Gy [5]. This is probably lower than the α/β [alpha/beta] ratio of the surrounding normal tissue such as the rectum, which is commonly thought to possess an α/β [alpha/beta] ratio of approximately 3 Gy [10]. This creates a scenario in which a greater differential tumor cell versus normal tissue sensitivity is created through the use of large dose per fraction radiotherapy treatment, potentially leading to higher cure rates or decreased normal tissue complication rates versus “conventional” fractionation radiotherapy regimens. Brenner and Hall conclude that in particular, this prostate cancer radiobiologic trait supports the use of high dose rate (HDR) brachytherapy, which inherently delivers large doses per fraction, or appropriately designed large dose per fraction external beam radiotherapy schedules [5].
The main imperfection in this theory is that not all investigators have concluded the α/β [alpha/beta] ratio of prostate cancer to be so low, with some deriving or postulating considerably higher values α/β [alpha/beta] ratio values of 3.7–5 Gy or more, leading to continued uncertainty [11–13]. Among other problems, there has been a relative paucity of >3 Gy/fraction data with requisite long-term follow-up in the literature to provide a full data base from which to derive a robust and accurate α/β [alpha/beta] ratio calculation for prostate cancer.
Assuming the low α/β [alpha/beta] rationale to be correct though, the SBRT modality is very well suited to deliver hypofractionated regimens, due to the inherent large dose per fraction provided by this approach. Although the α/β [alpha/beta] ratio of prostate cancer may well be lower than surrounding normal tissues, those normal tissues also have a relatively low α/β [alpha/beta] ratio, meaning they too are potentially damaged by large dose per fraction radiotherapy treatment. As such, tight margins and extreme accuracy are a necessary prerequisite when delivering hypofractionated radiation treatment. SBRT nicely fulfills this requirement, due to the very high spatial accuracy of a number of SBRT approaches [14–21].
Preceding modern SBRT, a different approach to hypofractionation has been utilized—high dose rate (HDR) prostate brachytherapy. This method has very high reported disease-free survival rates utilizing a variety of hypofractionation regimens, ranging from 3,800 cGy/4 fractions to 5,400 cGy/9 fractions as monotherapy, primarily (but not exclusively) against low-intermediate-risk lesions [22–25]. The HDR brachytherapy modality has also been used successfully as a prostate boost method, in conjunction with wide field pelvic radiotherapy, against a wider range of low-risk to high-risk prostate cancer cases, in which instance HDR brachytherapy is used to maximize radiobiologic potency against the primary lesion itself, with the wider coverage of conventional external beam radiotherapy applied to cover potential subclinical disease extension pathways beyond the prostate [26, 27]. There is some evidence that this combined modality approach has superior efficacy versus external beam monotherapy, even versus “dose escalated” external beam radiotherapy monotherapy [28, 29].
The emergence of SBRT represents a contemporary approach that potentially combines the better features of external beam radiotherapy and HDR brachytherapy; in that the SBRT modality maintains the noninvasive trait of external beam radiotherapy, yet with the capability to deliver “HDR-like” dose fractionation, dosimetry morphology and accuracy [30, 31].
The initial contemporary prostate SBRT regimen was described by King et al., using a hypofractionated schedule of 36.25 Gy/5 fractions, using the CyberKnife device as the delivery mechanism, against low- and low-intermediate risk cases, the first so treated in 2003. This dose regimen was selected to provide biologic equivalence to a conventionally fractionated external beam radiotherapy regimen of 74 Gy/37 fractions, assuming a prostate cancer α/β [alpha/beta] ratio of 3 Gy [14]. If the true α/β [alpha/beta] ratio of prostate cancer is lower, as is now fairly widely believed (but still not universally agreed), the biologic potency of this regimen may significantly exceed 74 Gy/ 37 fractions, and could in fact, exceed 90 Gy/45 fractions.
Considering the relatively narrow margins and high purported central biologic potency of SBRT, there are three different potential uses of this modality against prostate cancer:
SBRT used as monotherapy for clinically localized lesions that have a minimal risk of tumor extension beyond the immediate peri-prostatic region
SBRT used as a prostate boost, in conjunction with wider field “conventional” pelvic radiotherapy, against high-risk lesions that have a substantially greater risk of tumor extension beyond the immediate peri-prostatic region (i.e.—significant risk of positive seminal vesicles, lymph nodes)
SBRT used as a narrow margin salvage method used against recurrent prostate cancer—either following failed prior prostate radiotherapy (local retreatment) or against limited metastatic foci.
11.2.1 Case History
A 59 year old otherwise healthy gentleman presented in 2007 with a prostate-specific antigen (PSA) level of 5.4 ng/mL (elevated versus prior levels). Prostate biopsy dated 7/27/2007 revealed Gleason score 3 + 4 = 7 adenocarcinoma from 10 % of the left lobe and Gleason score 3 + 3 = 6 adenocarcinoma from 10 % of the right lobe (“overall” Gleason score 3 + 4 = 7). His prostate was palpably normal (Clinical stage T1c). His I-PSS score was 11/35 (c/w mild preexisting voiding symptoms) and his presenting Sexual Health Inventory Matrix (SHIM) score was 25/25, indicating full potency. He was very concerned about loss of potency with treatment. After careful consideration of a number of potentially curative surgical and radiotherapeutic treatment methods, he selected CyberKnife SBRT monotherapy as his treatment of choice. Due to relatively limited efficacy data in 2007, he agreed to receive his prostate SBRT treatment as a participant in an IRB-approved clinical trial [32].
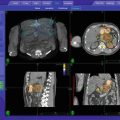
On September 7, 2007, he received 4 transperineally implanted intraprostatic gold fiducials to serve as his SBRT image guidance reference structure (Fig. 11.1). His prostate measured 59.6 cc on ultrasound imaging, with no other specific abnormally visualized. Fiducial-to-fiducial coregistered CT and MRI-based treatment planning was accomplished 1 week later, with a Foley catheter in place for both studies to identify the location of the urethra. The prostate and seminal vesicle bases were defined primarily from the MRI image set and contoured contiguously, with all contours also reconciled with the reference CT image set, to form the Gross Target Volume (GTV).
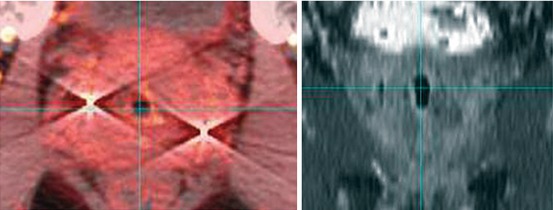

Fig. 11.1
Prostate fiducials as seen on CT-based (L panel) and MRI-based (R panel) images: Note fiducial prominence and streak artifact on the CT image, with comparatively less prominence and no artifact on the MRI image
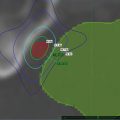
An asymmetric margin expansion was then applied to form a Clinical Target Volume (CTV) that covered the entire prostate and potential extracapsular extension contiguously. Specifically, this consisted of GTV + 2 mm on the right, GTV + 5 mm on the left (the side that harbored Gleason 7 disease and MRI-abnormality; felt to be at higher risk of extracapsular extension), contiguously also surrounding the seminal vesicle bases by 2 mm, and shaved to zero mm against the rectum. There was no further CTV to PTV expansion (CTV = PTV) (Fig. 11.2).
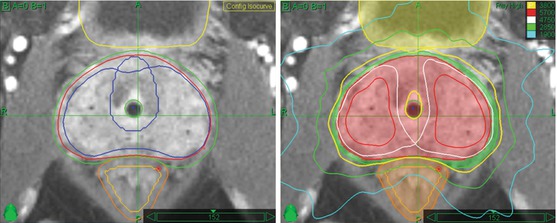

Fig. 11.2
Prostate CyberKnife SBRT contours and composite treatment plan: Note asymmetric peripheral margin expansion, with a larger left-sided margin expansion to accommodate the higher probability and potential magnitude of extracapsular tumor extension due to left-sided Gleason 7 disease. Note also the “shaving” of the PTV margin to zero at the prostate-rectal interface in the midline. Finally, note “HDR-brachytherapy-like” dose escalation in the extraurethral prostate, with central sparing and sharp posterior fall-off to limit urethral and rectal dose, respectively. The 100 % isodose line (yellow) follows the outer rectal wall while the 75 % isodose line (green) follows the rectal mucosa, which is defined as a 3 mm contraction from the rectal wall. The 125 and 150 % internal dose escalation isodose lines are displayed in white and red, respectively
Although it could be argued that at least a mm or two of extra CTV to PTV margin expansion could also be applied to account for issues such as image interpretation/registration uncertainty and potential interfraction/intrafraction prostate distortion, the 2 Gy/fraction radiobiological dose equivalent of the Virtual HDR protocol is 9,400 cGy for an α/β [alpha/beta] ratio of 3 Gy (a commonly assumed rectal α/β [alpha/beta] ratio value). As such, any added margin expansion is potentially injurious to adjacent tissue, and we thus choose to err on the side of “tight” margins on this protocol. The published sub-millimeter tracking accuracy of the CyberKnife delivery device and its ability to correct beam aiming in the translational and rotational dimensions (“6D”) further support the reasonability of “tight” margins when using this technology [14, 15].
He subsequently received protocol SBRT treatment on consecutive days, from 10/15/2007—10/18/2007, using the CyberKnife device as the SBRT delivery mechanism. The prescribed dose was 3,800 cGy/4 fractions, prescribed to the 49 % isodose line, creating an intraprostatic maximum dose (“Dmax”) of 7,755 cGy within the left lobe (“HDR-like” intraprostatic dose escalation) to a PTV that measured 148.2 cc,. Adjacent tissues (bladder, urethra, rectal wall and rectal mucosa) were also subject to “HDR-like” dose limitations per protocol (120, 120, 100 and 75 % of prescribed dose, respectively). Minor protocol deviations were accepted on the bladder, rectal wall and rectal mucosa Dmax levels to assure full PTV coverage, while the urethra dose level was fully protocol compliant.
Acutely, he had grade I GU and GI toxicity, including an I-PSS symptom score increase of 6 points and some rectal discomfort at the 2 week follow-up visit, fully resolved to baseline by the 1 month follow-up visit. There was no chronic GU or GI toxicity. His sexual potency was fully maintained, with a SHIM score of 25/25 at all short-term and long-term follow-up visits, out to 5 years.
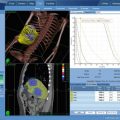
The PSA level steadily decreased from 5.4 ng/mL pre-treatment to 1.0 ng/mL by 6 months post-treatment, increasing to 2.3 ng/mL at the 1 year follow-up (“PSA bounce”), thereafter decreased to 0.4 ng/mL by the 2-year follow-up visit, followed by a second minor “PSA bounce” to 0.5 ng/mL at the 30 month follow-up, steadily decreasing after that to a final PSA nadir level of 0.1 ng/mL by the 4 year and 5-year follow-up visits. The PSA response curve for this case is illustrated in Fig. 11.3.
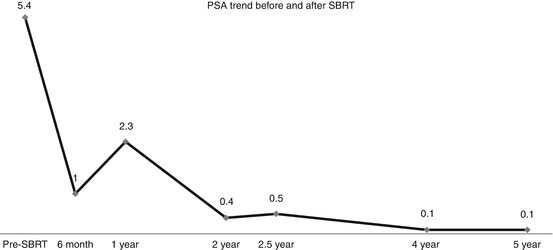

Fig. 11.3
Pre-treatment and follow-up PSA values, demonstrating common post-SBRT response kinetics: There is a rapid, early drop to 6 months, followed by a self-limited “PSA bounce” at a year, presumably reflecting post-SBRT delayed prostate inflammation, followed by resumption of a declining pattern with a second minor “PSA bounce” at 2.5 years, taking 4 years to achieve the final nadir PSA value
The SBRT planning MRI had shown a 61 cc prostate with diminished left lobe T2-weighted signal intensity and gadolinium hyperperfusion, c/w stage T2b disease. Follow-up MRI at 2 years revealed resolution of abnormalities and a prostate volume shrinkage to 35 cc (reduction of 43 %). He remains free of complications, with fully maintained potency function and improved voiding function (baseline I-PSS score of 11/35 pre-SBRT decreased to 2/35 at the 5 year follow-up visit). He will continue to have annual PSA-based follow-up visits.
11.3 Review of Literature (Review of the Evidence)
11.3.1 SBRT Monotherapy for Previously Untreated Cases
The predominance of published literature to date describes SBRT monotherapy against low- and intermediate-risk prostate cancer cases [33–35]. The initial cohort was recently updated by King et al. [33] In that study 67 low and low-intermediate-risk patients were treated with SBRT to a dose of 36.25 Gy/5 fractions, to a planning target volume (PTV) created by an anisotropic margin expansion of the prostate, by 3 mm posteriorly and 5 mm elsewhere, prescribed to cover >95 % of the PTV with the prescription dose, using the CyberKnife as the delivery device.
The 4-year actuarial biochemical disease-free survival was 94 % (median follow-up 2.7 years), with 2 biopsy proven local failures, and a reassuringly low rate of grade 3 or higher toxicity (3 % GU, 0 % GI). The few observed cases of grade 3 GU toxicity were preceded by urologic instrumentation. Grade 1–2 GU and GI toxicity was seen in 28 and 12.5 % of patients, respectively, with a statistically significantly lower incidence of low grade toxicity in patients that were treated every other day (q.o.d.) as opposed to daily (q.d.) (p = 0.001 for gastrointestinal and p = 0.007 for genitourinary). It was concluded that SBRT efficacy compared well with other treatments and that the incidence of severe toxicity was low, causing the authors to suggest that “current evidence supports consideration of stereotactic body radiotherapy among the therapeutic options for localized prostate cancer.”
A pooled dual institutional study by Freeman and King, restricted to a smaller cohort of 41 CyberKnife SBRT patients treated to 35–36.25 Gy/5 fractions with a significantly longer 5-year median follow-up was also reported. They reported similar findings—93 % 5-year biochemical disease-free survival and only a single case of grade 3 GU toxicity, with no Grade 3 or higher GI toxicity [34].
A different study reported by Katz et al., evaluated the efficacy of two different CyberKnife SBRT dose-fractionation regimens (35 Gy/5 fractions versus 36.25 Gy/ 5 fractions) in two groups of patients with predominantly low-risk prostate cases—41 consecutive patients treated to 35 Gy/5 fractions versus 41 matched subsequent cases treated to 36.25 Gy/5 fractions, with 54 and 48 months median follow-up in each group, respectively [35]. The crude biochemical disease-free survival rate measured 97.5 % with each dose regimen, with no significant difference in PSA nadir (0.1 ng/mL at 4 years) or toxicity rates. The incidence of late grade 2 urinary toxicity was 5 and 10 % in the 35 Gy versus 36.25 Gy, groups, respectively (p = NS), with an identical 5 % rate of grade 2 GI toxicity in each group. The authors concluded that “overall, the highly favorable PSA response, limited biochemical failures, limited toxicity, and limited impact on quality of life in these low- to low-intermediate-risk patients are supportive of excellent long-term results for CyberKnife delivered SBRT” and their work suggested that there is no efficacy loss with the lower dose level. It should be pointed out though, that “local relapse” may be a very late event, meaning their conclusion of equivalence is at best “hypothesis generating,” and requires longer-term confirmation before the efficacy of the lower dose arm may be considered truly identical to the more commonly used dose level of 36.25 Gy/5 fractions.
Based on ample successful brachytherapy precedent, a different and more aggressive prostate CyberKnife SBRT method and fractionation scheme, known as “Virtual HDR” SBRT, was described in an IRB-approved protocol in 2006, with comparative dosimetry analysis versus actual HDR brachytherapy published in 2008 along with very preliminary clinical results [30, 32]. Using this approach, prostate CyberKnife SBRT treatment plans are deliberately designed to mimic HDR brachytherapy as closely as possible; using an actual HDR brachytherapy schedule of 3,800 cGy/4 fractions, previously described by Grills et al. [22].
Beyond simply mimicking the dose-fractionation aspect of HDR brachytherapy, this method also applies substantially identical intraprostatic and periprostatic isodose morphology, including “HDR-like” urethra, bladder and rectal maximum “Dmax” dose limitation constraints. Additionally, to qualify as “Virtual HDR” a prostate SBRT treatment plan is required to produce significant intraprostatic dose heterogeneity, just as is seen with actual HDR brachytherapy, with intraprostatic Dmax dose escalation greater than 150 % of the prescribed dose, requiring prescription to an isodose line less than 67 %, and with the majority of such treatment plans prescribed to an isodose line of 50–55 %. This translates to a typical intraprostatic maximum dose level approaching twice the prescription dose level. This dose escalation region is designed to superimpose primarily over the peripheral zone of the prostate—the region that most frequently harbors the largest volume of intraprostatic cancer cell burden [33]. As imaging techniques such as multi-parametric MRI or evolving PET/CT methods more accurately define the “dominant intraprostatic lesion” (“DIL”), this technique may also be modified to restrict the intraprostatic dose escalation to the actual DIL, though the preponderance of “Virtual HDR” experience gained to date has been with the use of dose escalation to essentially the entire peripheral zone [36–39].
The Virtual HDR series was most recently updated in abstract form in 2012, reporting 51 patients (63 % low-risk; 37 % intermediate-risk), with a median follow-up of 3.5 years (range 6—60 months) [40]. Actuarial 4-year biochemical disease-free survival in this series is 98 % (both definitions), with a 100 % local control rate and a median 4-year PSA nadir of 0.1 ng/mL. There is no acute toxicity in this series greater than grade 2 in either the GU or GI domain. Late toxicity is primarily observed in the GU domain; with 18 and 6 % rates of late grade 2 and 3 GU toxicity, respectively. All observed cases of grade 3 GU toxicity are comprised of urethral strictures requiring surgical correction—a comparable incidence versus the predicate actual prostate HDR brachytherapy series upon which this series was based [41]. There is a statistically significant correlation of increased baseline I-PSS score with an increased rate of grade 2 or higher late GU toxicity, suggesting that caution is warranted in the use of this specific technique and dose-fractionation in patients that have significant preexisting obstructive uropathy (p = 0.0039; I-PSS score as continuous variable versus maximum grade GU toxicity—previously unpublished data). Corresponding rates of late grade 2 and 3 GI toxicity in this series are 2 and 0 %. Patient accrual is scheduled to continue in this series for the foreseeable future, and a larger multi-institutional series that began in 2007, evaluating the same technique, has also completed accrual [42].
The UCSF group has similarly described a “HDR-like” prostate CyberKnife SBRT approach, albeit with less extreme intraprostatic dose escalation versus the Virtual HDR method (UCSF series prescribes to the 60–80 % isodose line, equating to moderately less intraprostatic dose heterogeneity), using the same 3,800 cGy/4 fraction monotherapy fractionation scheme as described above, as well as a 1,900 cGy/2 fraction prostate boost in conjunction with wider field “conventional” pelvic radiotherapy to 45–50 Gy for patients felt to be at higher risk of extraprostatic disease [31]. This series describes 38 patients (20 SBRT monotherapy and 18 SBRT boost).
With a maximum follow-up of 43.5 months, the median observed PSA nadir in this series is 0.35 ng/mL, likely not the true nadir, as their median follow-up is only 18 months. Their late grade 2 and 3 GU toxicity rates measure 8 and 5 %, respectively, with a 3 and 0 % incidence of late grade 2 and 3 GI toxicity, respectively—extremely similar to the levels observed in the San Diego Virtual HDR series described above. The extra toxicity nuance added by the UCSF series is the finding of no apparent difference in the incidence of grade 2 or higher GU or GI toxicity whether this SBRT method is used as monotherapy or as a boost in conjunction with large volume “conventional” pelvic radiotherapy, though absolute numbers of patients in this series remains small, with less than mature follow-up, such that this has to be regarded as a “preliminary” finding of toxicity equivalence between methods.
Experience with gantry-based SBRT regimens as monotherapy for low- to intermediate-risk prostate cancer has also emerged, though the efficacy result seems less established with this approach, due to greater variability of reported outcomes. The initial gantry-based SBRT approach described in contemporary literature has been the so called “SHARP” (Stereotactic hypofractionated accurate radiotherapy of the prostate) regimen described by Madsen et al., in 2007 [43]. This method applied a dose of 33.5 Gy/5 fractions prescribed at the isocenter, using a fixed beam non-coplanar IGRT approach, treating the prostate with a 4–5 mm expansion margin from the prostate to the block edge (which equates to a far smaller margin between the prostate and “quasi full dose” 90 % isodose line). The authors assumed a prostate cancer α/β [alpha/beta] ratio of 1.5, equating to a 2 Gy/fx radiobiological dose equivalent of 78 Gy at the isocenter (author note—This decreases to a total “conventional” fractionation dose equivalent 64 Gy at the 90 % isodose line, which is at or barely beyond the margin of the prostate; lower still if the α/β [alpha/beta] ratio actually exceeds 1.5 Gy). Forty low risk patients were prospectively enrolled and evaluated in this study.
The biochemical disease-free survival rate of the “SHARP” study is unclear, as the result is substantially different depending on which specific definition of biochemical relapse is applied—70 % disease-free survival at 4 years using the ASTRO biochemical relapse-free definition versus 90 % using the Phoenix definition, with the higher biochemical relapse rate implied by the ASTRO definition potentially explained by “delayed PSA bounces” according to the authors. The most common PSA nadir level observed in this study was 0.6–1.0 ng/mL, with 74 and 32 % of all patients achieving a nadir level below 1.0 and 0.5 ng/mL, respectively. Comparison of the PSA nadir values in this series versus those reported in external beam radiotherapy literature caused the authors to conclude that this regimen likely has a biologic potency in the range of 73–78 Gy of “conventionally” fractionated radiotherapy.
Toxicity was reasonably low in this study for both the GU and GI domains. There was a single acute GU grade 3 toxicity and no delayed toxicity in either domain higher than grade 2, with 20 and 7.5 % delayed grade 2 GU and GI toxicities, respectively. The authors concluded that there is probably room for dose escalation. An update of this series with 5-year median follow-up was provided in abstract form in 2010, reporting 71 and 93 % 5-year biochemical relapse-free survival using the ASTRO and Phoenix definitions, respectively, with a median PSA nadir value of 0.65 ng/mL, implying no further degradation in efficacy to 5 years [44].
The substantial uncertainty in biochemical relapse-free survival with the SHARP regimen depending on relapse definition and the low DFS rate imputed by the ASTRO definition suggests the possibility that this regimen may not have an acceptable rate of efficacy. This concern is amplified by the fact that the suboptimal result in this series occurred in spite of restricting the treatment to low-risk patients. At best, the long-term efficacy of this specific SBRT regimen remains uncertain. Acknowledging this concern, the SHARP study authors have suggested consideration of dose escalation in both their initial and follow-up reports [43, 44].
A subsequent gantry-based prostate SBRT series described by the UT Southwestern group has employed a substantially more aggressive approach, sequentially evaluating dose levels of 45 Gy/ 5 fractions, 47.5 Gy/5 fractions and 50 Gy/5 fractions in cohorts of 15 low-risk and intermediate-risk patients, with escalation to the each succeeding dose level contingent upon the demonstration of reasonable acute safety of the previous dose level [45]. A total of 45 patients were described in their report. The treatment was delivered to a volume that included the prostate + 3 mm uniform margin expansion to form the PTV, with the dose prescribed to cover >95 % of the PTV, delivered using an axial plane IMRT method with daily image guidance, either by a helical Tomotherapy device or by a step and shoot MLC-based linear accelerator device.
At 12–30 months of follow-up, PSA control by the Phoenix definition (nadir + 2 ng/mL) is 100 %. The maximum grade 2 GU and GI toxicity incidence rates of 31 and 18 %, respectively, and maximum grade >3 GU and GI toxicity rates of 4 and 2 %, respectively; overlap the toxicity incidence of other contemporary radiotherapy series. This suggests a non-excessive severe toxicity rate in spite of aggressive SBRT dosing, with the caveat that a maximum follow-up of 30 months is inadequate to fully define the late toxicity incidence. As such, the late toxicity result of this approach must still be regarded as preliminary. The authors postulate that the relatively low toxicity incidence observed to date in spite of aggressive dosing, is due to tight margins, strict dose limitation of all adjacent normal tissues (bladder, rectum and urethra) and the use of an endorectal balloon to stabilize the rectum and displace its lateral and posterior walls out of the high dose volume. The study has now expanded to multiple centers and is ongoing.
Another gantry-based prostate SBRT series of 65 low-risk patients has been described in abstract form by Mantz et al., using a Varian Trilogy cone beam CT (CBCT) volumetric-guided IMRT approach, with Calypso electromagnetic tracking to provide 4D localization, with an action level of 2 mm [46]. They deliver a dose of 40 Gy/5 fractions (q.o.d. schedule) to the PTV (exact expansion from the prostate not described in the abstract). With 36 months of median follow-up, the PSA response is excellent (median PSA nadir 0.3 ng/mL at 3 years) and there is a zero incidence of reported grade 3 or higher toxicity in the GU or GI domains. As with other gantry-based reports, this series seems encouraging, with further follow-up and more complete reporting necessary to fully validate the results.
11.3.2 SBRT Radiobiology Potency Considerations
11.3.2.1 Intraprostatic Dose Considerations
When contemplating a specific dose fractionation regimen, it is useful to consider the “conventional fractionation” dose equivalent under various schedules and α/β [alpha/beta] ratio scenarios, outlined in Table 11.1. As revealed by this table, all SBRT dose fractionation scenarios are likely adequately powered if the α/β [alpha/beta] ratio of this disease is less than 2 Gy, whereas greatly differing degrees of efficacy loss are predicted under higher α/β [alpha/beta] ratio scenarios (e.g. if the α/β [alpha/beta] ratio of a lesion is actually 5 Gy instead of 1.5 Gy, the higher dose regimens are still likely efficacious, whereas the lower dose regimens are likely underpowered).
Table 11.1
RBE table comparing four popular prostate SBRT dose fractionation regimens, converted to 2 Gy/fx dose equivalent, with differing α/β [alpha/beta] ratio scenarios
Regimen | 3,500 cGy/5 fx | 3,625 cGy/5 fx | 4,000 cGy/5 fx | 3,800 cGy/4 fx |
|---|---|---|---|---|
α/β [alpha/beta] 1.5 Gy; 2 Gy/fx eq. | 8,400 cGy | 9,000 cGy | 10,800 cGy | 12,000 cGy |
α/β [alpha/beta] 3 Gy—2 Gy/fx eq. | 7,000 cGy | 7,400 cGy | 8,800 cGy | 9,400 cGy |
α/β [alpha/beta] 5 Gy—2 Gy/fx eq. | 6,000 cGy | 6,400 cGy | 7,400 cGy | 7,800 cGy |
11.3.2.2 Periprostatic Dose Considerations
In addition to considering the conventional fractionation dose equivalent required within the planning target volume, it is also useful to recall that although prostate SBRT coverage margins may be relatively sharper versus “conventional” radiotherapy approaches, there is still a non-trivial a dose gradient beyond the PTV, lending various degrees of effective “subclinical disease extension” coverage to the periprostatic region, such that there could be efficacy differences in various regimens based on the radiobiologic strength of lower isodose lines that extend beyond the prostate (e.g.—75 % of the prescription dose), with 75 % IDL conventional fractionation equivalents illustrated in Table 11.2. This table demonstrates that a regimen barely powered to control intraprostatic disease also loses efficacy beyond the prostate more rapidly versus more radiobiologically potent SBRT regimens. This implies that more conservatively dosed prostate SBRT regimens should be restricted to only those patients with the lowest risk of extraprostatic disease extension, while more potent SBRT regimens are more likely effective against a wider range of higher-risk periprostatic disease extension scenarios. An example of the extra periprostatic volume contained within the 75 % isodose line is illustrated in Fig. 11.4.
Table 11.2
RBE table comparing the 75 % isodose line potency of four popular prostate SBRT dose fractionation regimens converted to 2 Gy/fx dose equivalent, with differing α/β [alpha/beta] ratio scenarios.
Regimen | 3,500 cGy/5 fx | 3,625 cGy/5 fx | 4,000 cGy/5 fx | 3,800 cGy/4 fx |
|---|---|---|---|---|
75 % Value | 2,625 cGy/5 fx | 2,720 cGy/5 fx | 3,000 cGy/5 fx | 2,850 cGy/4 fx |
α/β [alpha/beta] 1.5 Gy; 2 Gy/fx eq.
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

|