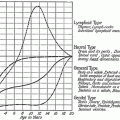
Fig. 1
Mean severity of symptoms at baseline and at weeks 3 and 5 during the course of RT (n = 419) (with permission from Hickok et al. 2005a)
The same University of Rochester research team recently utilized a modified version of the MDASI to assess the prevalence of multiple symptoms and their interference with daily life activities in patients undergoing radiotherapy. The primary goals were to determine the prevalence and the pattern of change in specific symptoms during and after radiation therapy and to evaluate the impact of symptoms on QOL and daily activities. All age groups and tumor types were included in the analysis. The investigators found a significant increase in the severity of almost all symptoms and their impact on QOL and daily activities. The overall symptom burden score (a sum of the individual symptom scores) significantly increased post-radiotherapy (Fig. 2). In addition, higher symptom burdens correlated with deficits in patients’ impressions of their own QOL.
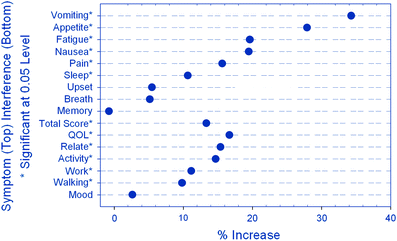

Fig. 2
Percentage increase in symptom interference following radiation therapy
The majority of previous symptom research has focused on individual symptoms, yet in reality patients often experience many different symptoms simultaneously. Research in patients undergoing radiotherapy has illustrated a high prevalence of symptoms that significantly impact QOL and daily activities. Moreover, high symptom burden and specific symptom clusters can have a deleterious effect on patient outcomes due to delays or discontinuation of treatment (Miaskowski et al. 2004). Given the negative effects of high symptom burden on daily activities and HRQOL, more research is necessary to evaluate the effect of interventions that target common symptom clusters rather than individual symptoms. Ideally, the development of such interventions will lead to a reduction of overall symptom burden in cancer patients.
3 Cancer-Related Fatigue
Cancer-related fatigue (CRF) is a global term used to refer to fatigue resulting from cancer and/or its treatments including surgically-induced, chemotherapy-induced, and radiation-induced fatigue. CRF is a multifaceted, psychosocial and physiological state characterized by an overwhelming sensation of exhaustion that is accompanied by a decreased capacity for mental and physical energy expenditure (Ahlberg et al. 2003; Barton-Burke and Barton-Burke 2006; Berger 1998; Broeckel et al. 1998; Carroll et al. 2007; Cella 1998; Cella et al. 1998, 2001, 2002; Curt et al. 2000; Escalante 2003; Hickok et al. 2003, Hickok et al. 2005b; Hofman et al. 2004, 2007; Irvine et al. 1991; Jacobsen et al. 2007; Jean-Pierre et al. 2007; Jereczek-Fossa et al. 2001; Kroenke et al. 1988; Mock 2004; Mock and Olsen 2003; Morrow 2007; Morrow et al. 2002, 2005; Mustian et al. 2007; National Comprehensive Cancer Network 2008; Piper et al. 1987; Portenoy and Itri 1999; Ryan et al. 2007). CRF is differentiated from the fatigue experienced by healthy individuals because of its severity, persistence, and impact on quality of life among cancer patients and survivors (Carroll et al. 2007; Hofman et al. 2007; Morrow 2007; Mustian et al. 2007; Ryan et al. 2007). Unlike the fatigue stemming from physical or mental exertion that can be relieved with rest or sleep, CRF is not alleviated by resting or sleeping (Carroll et al. 2007; Hofman et al. 2007; Morrow 2007; Mustian et al. 2007; National comprehensive Cancer Network 2008; Ryan et al. 2007). Cancer patients and survivors report that CRF begins with diagnosis, worsens during the course of treatment, and can persist for months and years after treatments are completed (Carroll et al. 2007; Hofman et al. 2007; Morrow 2007; Mustian et al. 2007; Ryan et al. 2007). CRF can also immerge for the first time months or years after treatments are completed (Carroll et al. 2007; Hofman et al. 2007; Morrow 2007; Mustian et al. 2007; National Comprehensive Cancer Network 2008; Ryan et al. 2007). As such CRF can be characterized as an acute or chronic side effect as well as an early or late side effect resulting from cancer and its treatments (Mustian et al. 2007).
From 60 to 100 % of cancer patients and survivors with a variety of diagnoses who are receiving different types of treatments report some degree of CRF, with 41 % or more indicating a severe level of CRF (0 = no CRF to 10 = CRF as bad as you can imagine; a score >7 = severe on this 11-point Likert Scale) during treatment (Ahlberg et al. 2003; Barton-Burke and Barton-Burke 2006; Berger 1998; Broeckel et al. 1998; Cella 1998; Cella et al. 1998, 2001, 2002; Curt et al. 2000; Escalante 2003; Hickok et al. 2001, 2003; Hofman et al. 2004; Irvine et al. 1991; Jereczek-Fossa et al. 2001; Kroenke et al. 1988; Mock 2004; Mock and Olsen 2003; Piper et al. 1987; Portenoy and Itri 1999). More than 80 % of cancer survivors report that the CRF they experience persists for months and even years after treatments are completed, with 17–38 % indicating that their CRF persists well beyond 6 months post-treatment (Barton-Burke and Barton-Burke 2006; Carroll et al. 2007; Frank-Stromberg and Wright 1984; Hofman et al. 2007; Jacobsen et al. 2007; Meyerowitz et al. 1983; Morrow 2007; Mustian et al. 2006; Mustian et al. 2007; Padilla and Grant 1985; Prue et al. 2006; Ryan et al. 2007). CRF is associated with a poorer prognosis among patients and often continues even when a survivor’s cancer is undetectable or in remission (Carroll et al. 2007; Hofman et al. 2007; Jacobsen et al. 2007; Jean-Pierre et al. 2007; Morrow 2007; Mustian et al. 2007; Ryan et al. 2007).
CRF has a considerable negative impact on an individual’s ability to perform important normal daily activities and overall QOL (Carroll et al. 2007; Hofman et al. 2007; Jacobsen et al. 2007; Jean-Pierre et al. 2007; Morrow 2007; Mustian et al. 2007, 2008; Ryan et al. 2007). This impact stems from the physiological and psychosocial signs and symptoms of CRF which typically include: anemia, hypothyroidism, shortness of breath, muscle atrophy, physical weakness, decreased aerobic capacity, decreased ability to walk, reduced capacity to bathe, dress or cook, sleep disruption, pain, self-reported tiredness, mood disturbance, depression, anxiety, hopelessness, negative outcome expectancies, impaired memory, the inability to concentrate, a reduction in patients’ ability to participate in leisure activities, a reduced capacity to sustain meaningful relationships and activities with families, a reduced ability to continue working and a reduction in engagement in social and other activities (Carroll et al. 2007; Hofman et al. 2007; Jacobsen et al. 2007; Jean-Pierre et al. 2007; Meyerowitz et al. 1979; Mock 2004; Morrow 2007; Morrow et al. 2002, 2005; Mustian et al. 2007, 2008; National Comprehensive Cancer Network 2008; Rhodes et al. 1988; Ryan et al. 2007). These signs and symptoms occur both during and after treatment for cancer (Carroll et al. 2007; Hofman et al. 2007; Jacobsen et al. 2007; Jean-Pierre et al. 2007; Morrow 2007; Mustian et al. 2007, 2008; Ryan et al. 2007). CRF is often reported as more distressing and having a greater negative impact on patients’ and survivors’ daily activities and QOL than other cancer-related symptoms such as vomiting, nausea, pain, and depression (Carroll et al. 2007; Curt et al. 2001; Hofman et al. 2007; Jacobsen et al. 2007; Jean-Pierre et al. 2007; Mock 2004; Morrow 2007; Morrow et al. 2002, 2005; Mustian et al. 2007, 2008; National Comprehensive Cancer Network 2008; Ryan et al. 2007). While great advances have been made in cancer treatments over the past decade, the negative impact of CRF is magnified because life expectancy has increased among cancer survivors. The experience of CRF is prolonged and there is no effective cure (Carroll et al. 2007; Hofman et al. 2007; Jacobsen et al. 2007; Jean-Pierre et al. 2007; Morrow 2007; Mustian et al. 2007, 2008; Ryan et al. 2007).
Cancer-related fatigue was first acknowledged as an official diagnosis in the International Classification of Disease (ICD-10) in 1998. The National Comprehensive Cancer Network (NCCN) published the first set of guidelines for the management of CRF in 2000. These guidelines were based on a synthesis of the best and most currently available research and clinical experience in oncology. These NCCN Practice Guidelines were recently updated in 2008 and provide concise, up-to-date, evidence-based and clinically-based recommendations for the management of CRF based on current knowledge of the symptom and development of new therapies (National Comprehensive Cancer Network 2008).
The current NCCN guidelines for the management of CRF (National Comprehensive Cancer Network 2008) suggest that clinicians frequently screen for CRF in patients with cancer and, when present, first treat possible contributing factors (e.g., pain, emotional distress, sleep disruption, anemia, nutrition, physical activity levels), including comorbid conditions (e.g., infection, cardiac dysfunction, pulmonary dysfunction, renal dysfunction, hepatic dysfunction, neurologic dysfunction, endocrine dysfunction, hypothyroidism), which is typically done via pharmacologic therapies (National Comprehensive Cancer Network 2008). Many patients with cancer continue to experience CRF even after successful clinical management of these contributing factors (Carroll et al. 2007; Hofman et al. 2007; Jacobsen et al. 2007; Jean-Pierre et al. 2007; Morrow 2007; Mustian et al. 2007, 2008; Ryan et al. 2007). Patients may also experience CRF in the absence of any clinically discernable contributing factors (Carroll et al. 2007; Hofman et al. 2007; Jacobsen et al. 2007; Jean-Pierre et al. 2007; Morrow 2007; Mustian et al. 2007, 2008; Ryan et al. 2007). In instances where no specific causal factors can be identified, or when the patient continues to have moderate-to-severe fatigue and/or persistent chronic fatigue after treatments for the cancer are complete and the other possible contributing factors have been clinically treated, the NCCN guidelines (National Comprehensive Cancer Network 2008) recommend that physicians consider both pharmacologic interventions and nonpharmacologic behavioral interventions including maintaining physical activity, psychosocial therapies, and other integrative therapies. Some of the most promising therapies that are still under investigation include exercise (Jacobsen et al. 2007; Mustian et al. 2007), psychosocial therapies (Jacobsen et al. 2007; Mustian et al. 2007), and Modafinil (Morrow et al. 2008).
Despite the existence of the NCCN guidelines and a growing body of literature that has identified some promising treatments, there is currently no evidence-based, effective cure for CRF, and there is no specific standard of care for CRF in the medical community. Moreover, very little attention has been given to the unique pathophysiological and treatment nuances of CRF as an acute versus chronic side effect or an early versus late effect. More scientific research is needed to discern the pathophysiology of all types of CRF and to develop effective cures for all types of CRF. This knowledge will provide the evidence upon which the standards of care for CRF in oncology will eventually be based.
4 Sleep Problems During Radiation Therapy
Relatively little attention has been devoted to sleep disturbance, one of the most distressing symptoms patients experience (Savard and Morin 2001). The spectrum of sleep disorders ranges from insomnia to sleep apnea, parasomnias, restless leg syndrome, and circadian rhythm disorders. Insomnia is defined as difficulty falling asleep, staying asleep or waking up earlier than intended, resulting in daytime complaints of fatigue or sleepiness for at least 1 month and not due to another mental disorder. Insomnia is prevalent; several epidemiological studies report that 15–20 % of the population has insomnia (Ohayon 2007). In general, population sleep loss and/or insomnia are associated with several negative physical and psychiatric consequences including fatigue, psychiatric illness (e.g., major depression and anxiety), physical complaints, substance abuse, reduced quality of life, and cognitive impairment (Breslau et al. 1996; Katz and Mchorney 2002; Lee et al. 2005; Leger et al. 2001, 2002; Ohayon and Zulley 2001; Walsh and Engelhardt 1999; Zee and Walsleben 2004). Some data also suggest that sleep difficulty may negatively influence cardiovascular conditions, reduce immune function, and affect the respiratory and musculoskeletal systems (Burgos et al. 2006; Katz and Mchorney 2002; Schwartz et al. 1999; Suka et al. 2003). Katz and Mchorney (2002) report that insomnia is independently associated with reduced health-related quality of life to the same extent as other serious chronic conditions such as diabetes and heart disease.
Although the full impact of insomnia on cancer patients and on disease progression is largely unknown, it is reasonable to assume that it would create similar adverse psychiatric and physiological consequences in cancer patients as in the general population. Insomnia may exacerbate other cancer-related symptoms (i.e., fatigue, nausea, depressive mood, pain, and/or reduced pain tolerance), and disrupted circadian rhythms may influence disease incidence, progression, and survival (Anderson et al. 2003; Fortner et al. 2002; Koopman et al. 2002; Mormont et al. 2000; Spiegel and Sephton 2002).
While the literature on the prevalence of insomnia in cancer patients is scant, two early studies found that the proportion of people who had difficulty staying asleep was significantly higher among cancer patients (40–45 %) than among healthy controls (14–25 %) (Kaye et al. 1983; Malone et al. 1994). Although both of these studies underscored the importance of studying sleep in cancer patients, the sample size in Kaye et al. (1983) was insufficient to draw any definitive conclusions about insomnia in cancer patients, and Malone et al. (1994) failed to undertake a detailed examination of sleep concerns.
A more recent study conducted by Savard and colleagues in 2001 focused specifically on the prevalence, clinical characteristics, and risk factors for insomnia in the context of breast cancer. They surveyed 300 breast cancer survivors with a median time since diagnosis of 49 months (Savard et al. 2001). Nearly 51 % reported insomnia symptoms, 19 % met diagnostic criteria for insomnia disorder, 33 % reported that insomnia occurred after they were diagnosed with cancer, and for 58 % of patients, insomnia became worse during the course of their cancer. This study was one of the first to note that insomnia prevalence is significantly higher in women with breast cancer than in the general population. Savard and colleagues also identified risk factors for the development of insomnia in breast cancer patients. The study found that unemployment, being on sick leave, and being widowed were significant contributors to the development of insomnia in this population. In addition, they reported that chemotherapy either caused or aggravated sleep difficulties. The authors hypothesized that the spike in symptoms during chemotherapy might be explained either by the antiemetic drugs used in treatment or the onset of menopause brought on by chemotherapy.
The most comprehensive insomnia prevalence study (Davidson et al. 2002) included 982 cancer patients (with a mean age of 65 years) with six different types of cancer (gastrointestinal, gynecologic, genitourinary, breast, lung, and nonmelanoma skin). Patients completed a “Sleep Survey” questionnaire that was used to evaluate the presence or absence of various sleep problems (e.g., insomnia, restless leg syndrome, fatigue) and to define their type of sleep problem (i.e., difficulty falling asleep, difficulty staying asleep, or waking up too early) (Davidson et al. 2002). Three hundred patients reported insomnia (30.5 %), and the majority (76 %) reported the greatest difficulty with frequent awakening. The authors found that the prevalence of insomnia was highest in the surgery group (45 %), followed by chemotherapy (34.5 %) and radiation (29.5 %); these differences, however, were not statistically significant. The authors also noted that cancer patients who reported marked fatigue were 2.5 times more likely to have insomnia than others.
The most recent study, conducted by Mao and colleagues, describes the symptom burden in cancer survivors (Mao et al. 2007). The author’s evaluated data collected from 1904 cancer survivors from the 2002 National Health Interview Survey. Nearly 52 % of the survivors were six or more years past their initial diagnosis of cancer, and nearly 30 % reported insomnia symptoms, compared to 17 % of healthy controls. Younger patients (age < 50 years) had an increased symptom burden compared to older patients (age > 64 years), and the adjusted odds ratios for insomnia were 2.7 and 1.44, respectively.
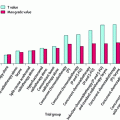
A recent study conducted by the URCC CCOP group (Palesh et al. 2007) surveyed 648 cancer patients (mean age = 61) who completed questionnaires evaluating fatigue, pain, depression, and difficulty sleeping. Sixty percent received chemotherapy and 40 % received radiation therapy. Participants rated the severity of their symptoms at their worst on an 11-point Likert Scale (0 = Not present to 10 = As bad as you can imagine) prior to treatment, during treatment, and 6 months following treatment. Patients reported significantly more sleep problems during treatment (median = 4) than either prior to or following treatment (both, median = 2). Women reported more sleep problems than men before, during, and after treatment (all p < 0.01). Younger patients (age ≤ 60) reported more problems with sleep at all time points (all p < 0.001). Patients receiving chemotherapy had more difficulties with sleep at all time points than patients undergoing radiation alone (all p < 0.001) (Fig. 3). These results show that women, younger patients, and patients undergoing chemotherapy versus radiation are at higher risk for developing sleep problems and, furthermore, that difficulties with sleep increase during cancer treatment.
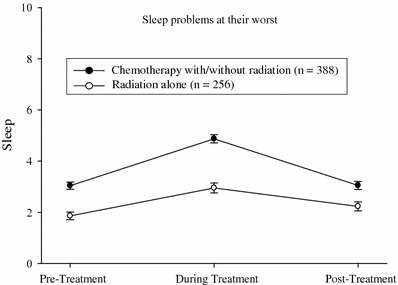

Fig. 3
Sleep problems by treatment type
All of these studies taken together indicate that insomnia is a prevalent and distressing symptom for cancer patients. Multiple studies indicate that insomnia rates among cancer patients are two to three times higher than rates in the general population. Increasing evidence suggests that cancer treatment might cause or exacerbate existing insomnia complaints and that the use of radiation and/or chemotherapy may cause these complaints to increase. To date, no definitive studies have evaluated insomnia in cancer patients undergoing radiation. It is still unclear whether insomnia precedes cancer or whether insomnia develops as a result of cancer diagnosis and/or treatment. Diagnosis and treatment of cancer and concerns about survival are severe stressors that in themselves might precipitate the development of insomnia; multiple additional biological and psychological “insults” (e.g., tumor biology, anti-cancer medications, early menopause, and fatigue) might also precipitate the development of insomnia symptoms. Given the high rate of insomnia in cancer patients, more investigation into its precipitating and perpetuating factors is needed in order to design effective interventions.
Within the general population, insomnia is highly treatable with pharmacological and psychological therapies (i.e., zolpidem and cognitive-behavioral therapy for insomnia). Several studies conducted in cancer survivors indicate that pharmacological and behavioral interventions are also effective in this group (Espie et al. 2008; Jacobsen et al. 1994; Savard et al. 2005). Additional research is needed to uncover physiological, psychological, and behavioral factors that contribute to the development of insomnia during radiation. Such studies will increase understanding of the natural course of insomnia disorder and allow the development of effective interventions.
5 Radiation Oncology and Psychological Functioning
A cancer diagnosis and its subsequent treatment represent major sources of stress that can lead to a variety of psychological difficulties (Dunn et al. 2004; Steginga et al. 1998). Two major themes characterize the psychological distress of cancer patients: first, the fear of the unknown, and second, the patient’s belief that the treatment will cause pain, discomfort, and/or adverse effects (Poroch 1995). While no consensus exists on the prevalence of psychological morbidity among cancer patients, seminal research in this area places the estimate as high as 47 % (Derogatis et al. 1983). Major depression, the most investigated psychiatric disorder in cancer, has been reported in 0–38 % of patients, and “depression spectrum disorders” in 0–58 % (Massie 2004). This wide range of prevalence estimates is largely due to the highly variable methods of assessment (e.g., self-report paper-and-pencil instruments, structured interviews, clinical interviews, and DSM diagnoses) and the differences in study design used by research teams since the 1960s. Investigation has also focused on anxiety disorders, with prevalence estimates ranging from 1.7–23 % and similar patterns of variability in study approach (Stark and House 2000).
Though other psychiatric illnesses (e.g., Posttraumatic Stress Disorder and Adjustment Disorder) have also received research attention, depression and anxiety are widely considered the most important comorbidities for cancer patients because of their potential for interference with treatment and association with poor health outcomes (Frick et al. 2007). Anxiety and stress have been associated with reduced survival and poorer prognosis, although the association is inconsistent (De Boer et al. 1999). There is also evidence that depression predicts progression and mortality and can affect immune function (Spiegel and Giese-Davis 2003). Psychological comorbidities may impair adherence to medical treatment, which could account for poorer outcomes (Spiegel and Giese-Davis 2003). Psychological distress among cancer patients has also been linked to impaired social functioning, nausea, and vomiting (Smith et al. 2003), all of which have a negative effect on quality of life (Frick et al. 2007; Smith et al. 2003).
In terms of psychological distress in response to radiation treatment (RT), an instructive review of the literature has been conducted by Stiegelis et al. (2004). They divided 45 studies, obtained through Medline and Psychlit databases between 1980 and 2002, into four categories representing studies that assessed psychological functioning: (a) prior to RT, (b) during RT, (c) after RT, and (d) using longitudinal designs. The studies included patients receiving both curative and palliative external radiation therapy with a range of cancer sites (e.g., breast, head and neck, prostate, bladder, cervix) and other adjuvant treatments (e.g., chemotherapy and hormone therapy).
Prior to RT, the studies reported that 10–20 % of patients had anxiety symptoms and only 1.5–8 % had depressive symptoms. The authors attribute the difference to anticipatory anxiety about treatment and its side effects prior to treatment and suggest that the absence of treatment side effects may account for relatively low levels of depression (Stiegelis et al. 2004).
During RT, anxiety symptoms were reported in 21–54 % of patients; these symptoms, however, showed relatively consistent declines as treatment progressed. For example, Rahn et al. (1998) showed that 40 % of patients were anxious on the first day of treatment, while only 19 % were still anxious by the last day. It is likely that patients’ anxiety decreased as they gained more familiarity with treatment. In contrast to the pre-treatment phase, depressive symptoms were relatively high during RT, with 12-31 % of patients reporting such symptoms.
Data were less consistent in studies examining anxiety and depression following radiation treatment. Reports of anxiety symptoms ranged from 0 to 52 % of patients, with assessments of anxiety covering time points from immediately after RT to as many as 13 years post-treatment. There was also significant variability in depression, ranging from 8 to 48 % of patients. However, most studies found that between 8 and 21.5 % of patients reported depressive symptoms. The authors suggest that, in some instances, differences in cut-off scores and response format may have elevated reporting (Stiegelis et al. 2004).
Longitudinal studies also showed inconsistencies, with some (Monga et al. 1999) finding an improvement in depressive symptoms over the course of RT and others (Chawla et al. 1999) finding a worsening of symptoms. Stiegelis and colleagues suggest that this difference may be attributable to varying baseline levels of psychological functioning and point to Andersen and Tewfik’s (1985) observation that: (a) patients with high levels of anxiety at baseline are more likely to see a substantial decrease in symptoms; (b) patients with low anxiety are likely to worsen; and (c) patients with moderate anxiety are unlikely to see much change in symptoms. However, the majority of both cross-sectional and longitudinal studies found declines in anxiety, depressive symptoms, and psychological distress after RT compared to the periods prior to and during treatment.
The literature has also addressed the particular distress that may accompany the end of RT (Deshields et al. 2005). This treatment termination distress may be occasioned both by the loss of support from medical staff, family, and friends once treatment is completed and by anxiety about returning to a regular routine (Culver et al. 2002; Lampic et al. 1994; Leedham and Ganz 1999). In a study of breast cancer patients at the end of RT, Deshields and colleagues found elevated levels of depression and somewhat diminished quality of life, but lower levels of anxiety compared to healthy population norms. Consistent with the studies reviewed by Steigelis et al., the authors speculate that anxiety associated with diagnosis and treatment had subsided by the end of RT. In addition, both depression and quality of life improved substantially within 2 weeks of the end of treatment.
Despite the evidence that depression, anxiety, and quality of life concerns are significant problems for cancer patients, especially in the early phases of diagnosis and treatment, barriers remain in terms of recognition and treatment of these symptoms in radiation oncology practice. These barriers include lack of communication about emotional issues between oncologists and their patients (Pollak et al. 2007), stigmatization and devaluation of mental health concerns and treatment (Holland 2003), belief that psychological problems are a “natural” part of the cancer experience (Spiegel 1996), and a tendency to dismiss somatic complaints thought to be associated with the cancer itself that may be indicative of psychological distress (Hahn et al. 2004; Jackson and Jackson 2007). Addressing these barriers to treatment of psychological problems in radiation oncology requires the concerted efforts of oncologists to properly assess these symptoms and to intervene when necessary.
Hahn et al. (2004) have demonstrated the effectiveness of the Beck Depression Inventory-II for identifying clinically depressed patients and strongly urge its routine use. Jackson and Jackson (2007) offer a helpful review of comorbid depression in adult oncology and note that both psychotherapeutic and pharmacologic treatments have been shown to be effective in this patient population. Similar brief measures and interventions are available for treatment of anxiety. In an historical retrospective on the field of psycho-oncology, Holland (2003) notes that the number of empirically supported psychosocial interventions has never been as great as it is now, making it relatively easy for radiation oncologists to refer patients for the appropriate treatment for psychological distress. These treatments not only increase patient quality of life but enhance compliance with treatment, which may affect survival.
6 Patient Information Needs During Radiation Therapy
One way to ameliorate psychological distress and improve quality of life for cancer patients is by providing appropriate information regarding the disease and its treatment. Educational and informational materials have been used to ease psychological distress for a variety of medical procedures including dental surgery, postoperative recovery, and MRI usage (Davis et al. 1994; Hartfield et al. 1982; Mogan et al. 1985; Selim 2001; Sime and Libera 1985). Early informational and educational programs were implemented in trials among cancer patients, with some success (Lilja et al. 1998; Mcquellon et al. 1998; Wells et al. 1995; Williams and Schreier 2004; Wilson et al. 2006). More recent research has shown that providing information and education to patients on both the disease and the course of treatment can improve psychological well-being (Helgeson et al. 1999; Helgeson et al. 2001).
The desire of cancer patients to be informed and educated about their treatment is well-documented. Information and education are particularly significant in the case of RT, because the treatment is frequently misunderstood by the general public (Chapman et al. 2003; Dunn et al. 2004). Research has shown that patients undergoing RT have fears directly related to the treatment itself and its perceived negative side-effects (Andersen et al. 1984; Frith 1991; O’rourke 1999). One study found that of 50 patients who were prescribed curative RT, the majority did not expect it to be effective (Haggmark et al. 2001). Of patients who considered themselves “well informed,” over two-thirds wanted more information, while only 2 % did not want any information on their treatment or its potential side effects (Cassileth et al. 1980). When providing informational materials to patients, clinicians must consider a number of factors (e.g., gender, age, ability to comprehend, family support) that influence patients’ preferences (Meredith et al. 1996). The lack of information and educational materials can intensify existing fears resulting from the initial diagnosis and can cause patients to misinterpret symptoms and the side effects of treatments (Cimprich and Ronis 2001; Peck and Boland 1977).
Harrison et al. assessed the information needs of breast cancer patients over their first course of radiation therapy (Harrison et al. 1999). Thirty-three women were interviewed about their informational needs and preferences during the first, third, and final week of treatment and again 1 month post-treatment. In general, patients had high information needs throughout the entire course of RT. This study also demonstrated that most women undergoing RT had a strong desire for information on both the disease and the treatment. Based on these findings, the authors recommend continually assessing the informational needs of patients throughout the course of their treatment.
There is also evidence that the provision of appropriate information has an impact on treatment-related side effects. A randomized trial conducted in 2002 examined the effects of information on the severity of treatment side effects in 152 RT patients (Kim et al. 2002). The intervention took place during the first and fifth radiation treatments and provided patients with specific, objective information about what to expect during treatment, while the control group was given only general information. The results demonstrated that the brief, targeted information was successful in reducing both fatigue and sleep problems among those who received it. This trial demonstrated that targeted information can have a positive impact on certain side effects, which may represent a method of improving quality of life among those receiving RT.
The timing of information dissemination can affect how patients use that information. A trial conducted in 2000 sought to determine the optimal timing of information provision to radiotherapy patients (D’haese et al. 2000




Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree