(1)
Department of Clinical Radiology, Amiri Hospital – Kuwait City, Kuwait City, Kuwait
7.1 Pleural Diseases
7.1.1 Pleural Effusion
7.1.2 Pneumothorax
7.1.3 Pleural Calcification
7.2.3 Types of Pneumonias
7.4 Sarcoidosis
7.4.1 Pulmonary Sarcoidosis
7.4.4 Cardiac Sarcoidosis
7.4.5 Neurosarcoid
7.5 Emphysema
7.7 Histiocytoses
7.7.3 Omenn Syndrome
7.7.4 Chédiak–Higashi Disease
7.8 Hemoptysis
7.8.3 Pulmonary Vasculitis
7.8.4 Cardiac Bronchus
7.8.5 Dieulafoy Disease
7.10.4 Cheyne–Stokes Respiration
7.1 Pleural Diseases
The pleura are composed of two layers, parietal and visceral layers, separated by a pleural space. The parietal pleuron is supplied by systemic vessels and drains into the right atrium via the azygos, hemiazygos, and internal mammary veins. The visceral pleuron is supplied by bronchial and pulmonary vessels and drains into the pulmonary veins.
The pleural space normally contains interstitial fluid (1–5 mL) that is cleared by the parietal pleural lymphatic vessels. There is no direct communication between the visceral pleura lymphatics and the pleural space.
The pleura appear normally on radiographs only when the X-ray beam is tangentially set on the film. On radiographs, the pleura appear as fissures and junctional lines. Fissures are made up of two layers of visceral pleura. The normal parietal pleuron is never visualized on posteroanterior (PA) radiographs.
Different pathological conditions affecting the pleura can be diagnosed with confidence by PA chest radiographs alone. This topic discusses the main pathological pleural conditions with their typical radiologic manifestations.
Pleural Effusion
Pleural effusion is a condition characterized by abnormal fluid collection between the parietal and visceral pleura (excess pleural space fluid). The pleural fluid can be water (edematous effusion), blood (hemothorax), pus (empyema), tumor cells (malignant pleural effusion), or lymph (chylothorax).
Pathologically, pleural effusion is divided into serous or exudative according to the protein content after lab analysis. Serous plural effusion contains little protein content (<2.5 g/dL) and usually arises due to systemic disease like cardiac failure, nephrotic syndrome, or liver failure. Exudative pleural effusion contains high protein count (>2.5 g/dL) and usually arises due to inflammatory or infectious process like tuberculosis, malignancy, and acute pancreatitis.
Disruption of the thoracic duct due to lymphoma or a tumor can cause lymphatic blockage and leakage into the pleural space causing chylothorax. Malignant effusion typically results from metastasizing of the malignant cells into the pleural cavity via the parietal pleura lymphatics, and it is often massive.
Bronchopleural fistula is a condition characterized by opening of a bronchus into the pleural space. It can develop occasionally following thoracic surgery, infection, medical intervention, or malignancy. Bronchopleural fistula is seen in 2–3 % of postpneumonectomy cases.
Signs on Chest Radiographs
Obliteration of the lateral costophrenic angle with a meniscus like arc at the interface between the fluid and the chest wall in PA radiographs (Meniscus sign) (Fig. 7.1.1).
Obliteration of the posterior costophrenic angle in lateral radiographs (Fig. 7.1.1). This angle is more sensitive to plural effusion collection due to gravity effect. Up to 50 mL of fluid is necessary to obliterate the posterior costophrenic angle, and 200 mL is necessary to obliterate the lateral costophrenic angle.
Subpulmonic pleural effusion (SPE) is a pleural effusion that occurs below the lungs at the diaphragmatic surface. SPE does not obliterate the costophrenic angle, but it distorts the shape of the diaphragmatic dome, giving the impression of raised hemidiaphragm. You can suspect SPE in the left lung when the space between the gastric bubble and the lower lung margins increases up to 3 cm instead of usual few millimeters. Beside the raised hemidiaphragm, the lung appears to end early on PA radiographs (Fig. 7.1.2).
Encysted (loculated) pleural effusion is a localized encysted fluid at the fissures between lobes of the lung. It occurs usually at the right lung’s minor fissure, and it has biconvex contour mimicking a mass (Fig. 7.1.3). Very rarely, a benign form of mesothelioma can grow along the major or minor fissures mimicking encysted pleural effusion, a condition known as pseudotumor.
Parapneumonic effusion is an effusion that develops adjacent to pneumonias (empyema). Almost 30 % of patients with pneumonia develop pleural effusion and usually resolve with antibiotic therapy.
Mediastinal pleural effusion is a fluid collection around the mediastinum. It is an unusual condition, and when it occurs, it forms silhouette sign along the mediastinal borders causing mediastinal widening. Silhouette sign is a term used to describe any opacity within the chest radiograph that obliterates a mediastinal border.

Fig. 7.1.1
Posteroanterior (a) and lateral (b) chest radiographs in two different patients with pleural effusion show meniscus sign with right pleural effusion obliterating the lateral costophrenic angle (arrowhead) in (a) and pleural effusion obliterating the posterior costophrenic angle in (b) (arrow)

Fig. 7.1.2
Posteroanterior chest radiograph of a patient with right subpulmonic pleural effusion (SPE) shows raised hemidiaphragm, and the lung seems to end early (arrowhead)

Fig. 7.1.3
Posteroanterior (a) and lateral (b) chest radiographs show right-sided encysted pleural effusion (arrowheads)
Signs on US
Pleural effusion appears as anechoic or hypoechoic collection that lies between the echogenic line of the visceral pleura and lung (Fig. 7.1.4).


Fig. 7.1.4
Transverse ultrasound image shows right-sided pleural effusion (arrowhead). The diaphragm can be visualized as a hyperechoic line separating the right lung base from the liver (arrow)
Signs on CT
Serous pleural effusion is visualized as a crescent peripheral area with CT water density. Exudative effusion can be hyperdense.
Empyema characteristically demonstrates thickened parietal/visceral pleura (e.g., > 2 mm) with effusion in between (split pleura sign) (Fig. 7.1.5). Enhancement of both pleura occurs in 80–100 % cases after contrast injection. Multiple gas pockets within the empyema may be seen.
Bronchopleural fistula occurs when a bronchus opens into the pleural space due to lung parenchymal destruction (e.g., pneumonia with empyema formation). It is seen as pleural effusion with air–fluid level on radiographs or HRCT (Fig. 7.1.6).

Fig. 7.1.5
Posteroanterior chest radiograph (a) and axial chest CT (b) of a patient with huge left-sided empyema show split pleura sign in (b), with thickened, enhanced pleura with effusion in between (arrowheads)

Fig. 7.1.6
Axial chest CT shows huge right bronchopulmonary fistula
Differential Diagnoses and Related Diseases
Meigs’ syndrome is a disease characterized by ascites, pleural effusion, and one of the following ovarian tumors (fibroma, thecoma, granulose cell tumor, or Brenner’s tumor). In contrast, Pseudo–Meigs’ syndrome is defined as ascites, pleural effusion, and ovarian tumor other than the ones mentioned previously. The absence of malignant cells from the ascites or the pleural effusion is mandatory for the diagnosis of Meigs’ syndrome. Typically, the ascites and the pleural effusions resolve after tumor resection. Meigs’ syndrome often occurs in postmenopausal women.
Yellow nail syndrome is a rare disease characterized by extremities lymphedema and thickened, slowly growing, yellowish-green nails that are excessively curved from side to side (Fig. 7.1.7). The disease is commonly accompanied by idiopathic pleural effusion, chronic bronchiectasis, chronic sinusitis, and lymphedema of the face. Yellow nail syndrome may be accompanied by rheumatoid arthritis or thyroid disease. The disease is believed to be caused by hypoplasia, atresia, or varicosity of the lymphatics.

Fig. 7.1.7
An illustration demonstrates the yellowish-green nails of the yellow nail syndrome
Pneumothorax
Pneumothorax is a condition characterized by the presence of air between the parietal and visceral pleura. There are three types of pneumothoraces:
Primary (spontaneous) pneumothorax: this type occurs without a defined cause and mainly seen in young males who are tall, thin, and smokers. Primary pneumothorax is attributed to rupture of subpleuritic blebs at lung apices according to some investigators.
Secondary pneumothorax: this type occurs usually after penetrating trauma, ruptured bulla, or an interventional thoracic procedure (e.g., lung mass biopsy).
Tension pneumothorax: this type occurs when the air collection within the subpleural space is large enough to push the mediastinum to the other side, interfering with blood circulation within the major vessels.
Up to 40 % of pneumothoraces may not be detected by chest radiographs. CT is 100 % sensitive for detection of pneumothoraces. When pneumothorax opens into the mediastinum, a pneumomediastinum develops. Pneumomediastinum is characterized by the presence of air around the mediastinal structures.
Sign on Radiograph
Thin visceral pleural line: it is visible on radiographs. The line is outlined by air with the absence of the peripheral vasculature laterally and lung tissue with possible increased density due to collapse, medially (Fig. 7.1.8). Lung apices are the best sites checked for early detection of pneumothorax.
Deep sulcus sign: the costophrenic angle deepens at the site of the pneumothorax (Fig. 7.1.9). It is seen in pneumothorax with large air collection.
Tension pneumothorax: it is seen as complete collapse of the lung and shift of the trachea and mediastinum to the contralateral (other side) collapsed lung (Fig. 7.1.10).
Pitfall: a skinfold and underlying clothing can mimic a pneumothorax (Fig. 7.1.11). Always correlate the radiological findings with the patient history and current status.
Pneumomediastinum: it is detected when the mediastinal structures are surrounded by dark radiolucent line of air (Fig. 7.1.12).

Fig. 7.1.8
Posteroanterior chest radiograph shows right pneumothorax with lung collapse (arrowhead)

Fig. 7.1.9
Posteroanterior chest radiograph shows right-sided pneumothorax (arrowhead) with right deep sulcus sign (arrow)

Fig. 7.1.10
Posteroanterior chest radiograph of a child with right tension pneumothorax shows mediastinal shift toward the left side

Fig. 7.1.11
Posteroanterior chest radiograph (a) and axial chest CT (b) of a patient with skin flap that appears as right-sided pneumothorax on chest radiograph (arrowheads), while on the HRCT, no pneumothorax is detected

Fig. 7.1.12
Posteroanterior chest radiograph (a) and axial chest CT (b) of a patient with pneumomediastinum show air around the heart and the mediastinal structures (arrows)
Pleural Calcification
Pleural calcification can be seen following chronic pleural damage. Pleural calcification can be unilateral or bilateral. Unilateral pleural calcification occurs usually as a late complication of empyema, hemothorax, fungal infection, or tuberculosis. Bilateral pleural calcification is commonly caused by asbestosis
Asbestosis is a pathological condition that results from previous exposure to asbestos. Pleural plaques are the commonest manifestation of asbestosis, and they usually develop 20–30 years after the exposure to asbestosis. They are composed of focal areas of parietal pleural thickening with dense hyaline collagen. Mesothelioma is an uncommon primary tumor of the serosal lining of the pleura or peritoneum. Only 5–7 % of patients with asbestosis develop mesothelioma. Mesothelioma has poor prognosis, with survival rate of 12 months. Bronchogenic carcinoma develops in 25 % of cases of asbestosis, and it is the main cause of death.
Signs on Chest Radiographs
Pleural plaques are seen as smoothly demarcated, well-defined opacities (in profile) or faint, ill-defined plaques (en face), in a bilateral fashion. Unilateral pleural plaques may be seen in 25 % of cases and usually located on the left side. The plaques usually are <1 cm in thickness and seen parallel to the chest wall.
Pleural calcification is seen in 15–25 % of cases after a latency period of 30–40 years (Fig. 7.1.13). Diaphragmatic calcification is pathognomonic finding of asbestosis.
Asbestos-related diffuse pleural thickening is a bilateral thickening involving at least 25 % of the chest or 50 % if unilateral, plus pleural thickness >5 mm at any site. The diffuse pleural thickening can affect the visceral layer and the parenchyma below, causing “fluffy fibrous strands.”
Round atelectasis is seen as a pleural mass (3–5 cm) that abuts over the pleura with a curvilinear tails entering the mass (comet tail sign). The curvilinear densities are composed of fibrosed vessels, and the mass is commonly visualized at the base of the lungs (Fig. 7.1.14). Air bronchograms within the mass are common.
Malignant mesothelioma is visualized as visceral or parietal pleural nodules that are indistinguishable from pleural metastases. The most common manifestation of malignant mesothelioma is a unilateral massive pleural effusion.

Fig. 7.1.13
Posteroanterior chest radiograph of a patient with asbestosis shows bilateral calcified pleural plaques

Fig. 7.1.14
Axial chest HRCT illustration demonstrates round atelectasis with comet tail sign (arrowhead)
Signs on HRCT
Pleural plaques appear as well-circumscribed areas of pleural thickening separated from the underlying ribs by thin layer of fat. The edges of the pleural plaque are typically thicker than its center.
Malignant mesothelioma is visualized as nodular pleural thickening (94 %) that commonly involves the lung bases (50 %) (Fig. 7.1.15). Diaphragmatic involvement (80 %), pleural calcification (20 %), and pleural effusion (80 %) are other common features.

Fig. 7.1.15
Posteroanterior chest radiograph (a) and axial chest CT (b) of a patient with malignant pleural mesothelioma show nodular thickening of the pleura in the right lung field with extension toward the apex in (a) and (b). Notice the nodular mass that follows the pleural distribution in (b) (arrowhead)
Further Reading
Akira M, et al. Asbestosis: high-resolution CT-pathologic correlation. Radiology. 1990;176:389–94.
DeCoste SD, et al. Yellow nail syndrome. J Am Acad Dermatol. 1990;22:608–11.
Gallardo X, et al. Benign pleural diseases. Eur J Radiol. 2000;34:87–97.
Goyal M, et al. Malignant pleural mesothelioma in a 13-yearold girl. Pediatr Radiol. 2000;30:776–8.
Nicholas G, et al. Asbestosis and malignancy. AJR. 1967;100:597–602.
Nimkin K, et al. Localized pneumothorax with lobar collapse and diffuse obstructive airway disease. Pediatr Radiol. 1995;25:449–51.
O’Lone E, et al. Spontaneous pneumothorax in children: when is invasive treatment indicated? Pediatr Pulmonol. 2008;43:41–6.
Qureshi NR, et al. Imaging in pleural disease. Clin Chest Med. 2006;27:193–213.
Sacco O, et al. Yellow nail Syndrome and bilateral cystic lung disease. Pediatr Pulmonol. 1998;26:429–33.
Váuez JL, et al. Pneumomediastinum and pneumothorax as presenting signs in severe Mycoplasma pneumoniae pneumonia. Pediatr Radiol. 2007;37:1286–8.
7.2 Alveolar Lung Diseases
Alveolar lung diseases (ALD) are group of disorders characterized by pathological insult involving mainly the alveoli. The alveoli can be imagined as an empty cup, and alveolar diseases are classified according to the content of this cup. Alveolar diseases are characterized by filling of the alveoli with materials that impede its normal physiological function (ventilation). Alveolar diseases can be localized (focal) or diffuse. Names of the conditions depend upon the content of the material filling the alveoli.
Types of Alveolar Lung Diseases
Alveoli filled with serous fluid: cardiogenic and noncardiogenic edema
Alveoli filled with blood: pulmonary hemorrhage, commonly due to vasculitis (e.g., Churg–Strauss syndrome)
Alveoli filled with pus: pneumonia
Alveoli filled with proteins: alveolar proteinosis and amyloidosis
Alveoli filled with malignant cells: bronchoalveolar carcinoma
Alveoli filled with calcium: alveolar microlithiasis
Pulmonary Edema
The alveoli are the main units for respiratory–blood ventilation and oxygenation and normally are full of air on inspiration. You can think of the alveoli as an empty cup, and any pathological condition that fills this cup will form a pathological condition according to the cup content. Pulmonary edema arises due to alveolar filling with serous fluid (water).
Pulmonary edema can be either due to cardiac disease (cardiogenic) or other conditions (noncardiogenic). Most cases of noncardiogenic pulmonary edema are due to acute respiratory distress syndrome (ARDS).
Cardiogenic pulmonary edema is commonly seen with heart failure. It starts as an interstitial edema before it turns into alveolar edema, because the pulmonary veins lie in the interstitium. As the hydrostatic pressure within the veins rises, they leak into the interstitium first and then progress to fill the alveoli. This process is rapid, and only very early edema can be seen as a pure interstitial linear pattern in chest radiographs.
Noncardiogenic pulmonary edema has the same radiographic features as the cardiogenic pulmonary edema, but the causes are different: ARDS, chemical pneumonitis, drug-induced pulmonary edema, and transfusion reaction are the most common causes for noncardiogenic pulmonary edema. ARDS is a situation where an alveolar capillary injury occurs as a result of variety of causes (e.g., sepsis). Chemical pneumonitis is a pulmonary edema that occurs due to inhalation of noxious chemical substance such as ammonia, smoking inhalation, near-drowning situations, and gastric acid aspiration. The mechanism of pulmonary edema is the result of one of the three mechanisms: irritation of the tracheobronchial tree that leads to inflammation and pulmonary edema formation; absorption of the noxious material from the respiratory tract, which can affect the lungs directly by its metabolites; and asphyxiation due to inhalation of high concentration of the noxious material that will displace oxygen from the blood and cause tissue hypoxia. Drug–induced and transfusion reactions pulmonary edema arise due to anaphylactic lupus-like reaction formation. The radiographic picture cannot be differentiated from ARDS unless you have history of drug ingestion or recent transfusion reaction. Classic examples of drugs causing pulmonary edema are heroin, aspirin, and penicillin. Negative pressure pulmonary edema is a term used to describe noncardiogenic edema that arises due to acute airway obstruction (type 1) or after the relief of chronic airway obstruction (type 2).
Signs on Radiograph
There is a centrally located, bilateral, symmetrical diffuse alveolar opacities emitting from the helium and spares the periphery (butterfly or batwings sign) (Fig. 7.2.16). Usually, pulmonary edema causes homogenous opacities, but sometimes they can cause nodular or blotchy opacities.
Cardiomegaly and signs of congestive heart failure (e.g., congested pulmonary vessels).
Kerley lines represent thickening of the interlobar septae. Lung lymphatics and veins run in the interstitium, leakage of the veins (edema), or tumor infiltration of the lymphatics (lymphangitis carcinomatosis) can result in thickening of the interlobar septa, which are called Kerley lines. Kerley A lines are long lines located near the lung hilum and extend obliquely near the bronchoarterial bundle into the peripheries. Kerley B lines are short white lines seen perpendicular to the pleural surface at the lung bases, commonly near the costophrenic angles (Fig. 7.2.17). Kerley C lines are a mixture between the two lines resulting in a reticular pattern.
Air-bronchogram sign is a sign seen when the alveoli are filled with fluid and the terminal bronchioles and bronchi are devoid of fluid (filled with air). The bronchioles appear as radiolucent lines within whitish radio-opaque opacities (Fig. 7.2.18). This sign is specific for alveolar disease, but nonspecific for the cause. Pulmonary edema, pulmonary hemorrhage, pneumonia, and alveolar carcinoma all look the same on radiographs. All appears as ALD with air bronchogram. The medical history plays a very important role in differentiating these conditions because the radiographic signs can be nonspecific.
In blood diversion, normally, the upper lobe vessels are not visualized on radiographs, and the lower lobe vessels are mildly dilated and visible due to the gravity effect in upright posteroanterior (PA) radiographs. In cases of cardiac diseases and pulmonary hypertension, the upper lobe vessels will be as wide as the lower lobe vessels in upright radiographs. Note that the upper lobe vessels can be seen dilated normally in supine (lying) chest radiographs (e.g., in intensive care unit radiographs).
Silhouette sign refers to a patchy, ill-defined radio-opaque shadow that obscures part of the normal mediastinal configuration.

Fig. 7.2.16
Anteroposterior plain chest radiographs in two different patients show bilateral symmetrical pulmonary edema with bat wings appearance in (a), and bilateral, almost symmetrical pulmonary hemorrhage in (b). Notice that without history, you cannot differentiate pulmonary hemorrhage from pulmonary edema based on radiographic presentation alone

Fig. 7.2.17
Posteroanterior plain chest radiograph shows Kerley B lines (arrowheads)

Fig. 7.2.18
Posteroanterior plain chest radiographs in two different patients with pneumonia show pneumonic lung patch with air columns within the patchy due to unaffected bronchi in (a) (arrowhead) and pneumonic lung patch with no air-bronchogram sign in (b). Patient (a) presents with airspace pneumonia, whereas patient (b) presents with bronchopneumonia
How to Differentiate Between Cardiogenic Edema from ARDS on Plain Chest Radiographs?
ARDS usually has a normal heart size, while cardiogenic pulmonary edema shows signs of heart failure.
ARDS usually affects peripheral lung field more than central, whereas cardiogenic edema typically starts from the center to the periphery.
ARDS usually has no Kerley B lines.
Pneumonia
Pneumonia is a condition characterized by an infectious inflammation of the lung parenchyma and deposition of pus within the alveoli. Pneumonia can be caused by bacteria (e.g., methicillin-resistant Staphylococcus aureus (MRSA)), fungi (e.g., Pneumocystis carinii), and viruses (e.g., Cytomegalovirus (CMV)).
Patients with pneumonia present with dyspnea, purulent sputum, fever, tachycardia, and maybe hemoptysis (e.g., tuberculosis). Complications of pneumonia include lung abscess formation, septicemia, and empyema. Rarely, arthritis and neurological symptoms may be encountered in atypical pneumonias (e.g., Mycoplasma pneumonia).
Pneumonias are divided into “typical pneumonia,” which is caused by Streptococcus pneumoniae (pneumococcus), and “atypical pneumonia,” which is caused by any pathogen that is not pneumococcus. Typical pneumonia is clinically dominated by respiratory symptoms, whereas atypical pneumonia clinically is dominated by symptoms of fever and malaise more than the respiratory symptoms.
Types of Pneumonias
Airspace pneumonia (lobar pneumonia): in this type, the infection is confined to a single lobe. There is usually one patch filling the whole affected lobe. This type is seen with pneumococcus, Legionella, Pseudomonas, and primary tuberculosis infection. Lobar pneumonia is characterized by an “air-bronchogram sign.”
Bronchopneumonia: this type is characterized by an infection that starts in the bronchioles and small bronchi walls and then spreads to the alveoli. This type is seen with Staphylococcus aureus, Haemophilus influenza, and Mycoplasma pneumonias.
Interstitial pneumonia: this type is characterized by an infection that involves the interstitial septa and giving reticular interstitial pattern on chest radiograph. This type can be seen with viral infections like influenza virus and varicella-zoster virus (VZV) and Mycoplasma infections (30 % of cases).
MRSA is a serious infection with antibiotic-resistant staphylococci. MRSA is categorized as community-acquired, nosocomial, and healthcare-associated infection. MRSA is the leading cause of nosocomial and healthcare-associated bloodstream infection, globally. Also, it is responsible for 30–50 % of ventilator-associated pneumonia. MRSA causes metastatic foci of infections in 30 % of cases into the lungs, liver, kidneys, heart valves, and joints. Most community-associated MRSA strains carry the Panton–Valentine leukocidin (PVL) gene, which is rarely found in the hospital-acquired MRSA or the normal strain of S. aureus. PVL toxin is a potent lethal factor to neutrophils, which causes tissue necrosis and severe necrotizing pneumonia. MRSA pneumonia is more frequently associated with sepsis, high-grade fever, hemoptysis, pleural effusion, and death compared to PVL-negative S. aureus. MRSA pneumonia can result in the formation of pulmonary cavitary infiltration due to the development of necrotizing pneumonia. The development of MRSA necrotizing pneumonia should be suspected in a young patient presenting with hypoxia, hemoptysis, and single or multiple cavitary lung lesions.
Viral pneumonias are characterized by several pathologies that include bronchiolitis, tracheobronchitis, and classical pneumonia. Viruses that attack immunocompetent patients include influenza viruses, Epstein–Barr virus, and adenoviruses. Viruses that attack immunocompromised patients include measles virus, VZV, and CMV. Measles virus attacks usually children due to immunosuppression or vaccine failure. VZV pneumonia is a common complication of VZV septicemia in children with a mortality rate of 9–50 %. Up to 90 % of VZV pneumonia cases are seen in patients with lymphoma or immunosuppression. CMV pneumonia is commonly seen in transplant patients and immunocompromised patients. Patients may develop severe necrotizing pneumonia in spite of antiviral therapy.
Differential Diagnoses and Related Diseases
Hyperimmunoglobulinemia E syndrome (Job’s syndrome) is a rare condition characterized by marked elevation of serum IgE levels against S. aureus, resulting in decreased production of antistaphylococcus IgG. The patient with this syndrome presents with frequent attacks of S. aureus pneumonia, pustular dermatitis, eczema, and sinusitis. Formation of chronic lung abscesses is a common feature on radiographs.
Signs on Radiograph
Radio-opaque patches with an air-bronchogram sign (Fig. 7.2.18).
For bulging fissure sign, some infections will increase the volume of the lobe involved, causing the adjacent fissure to bulge (commonly the transverse fissure) (Fig. 7.2.19). This sign is classically seen in Klebsiella pneumonia.
In bronchopneumonia, there are multiple patchy infiltrations of the lung with or without segmental lobe atelectasis (if the bronchus is totally obstructed) (Fig. 7.2.18).
Interstitial pneumonia shows nonspecific linear or reticular interstitial lung pattern. Correlation with history and laboratory findings is essential to establish the diagnosis.
Viral pneumonias can appear as poorly defined nodules (4–10 mm in diameter), with lung hyperinflation due to bronchiolitis.
Measles pneumonia shows mix pattern of reticular interstitial pattern with patchy pneumonia (Fig. 7.2.20). Hilar lymphadenopathy may be associated.
VZV pneumonia appears as multiple, ill-defined micronodules (5–10 mm) (Fig. 7.2.21). The lesions may calcify persisting as well-defined, randomly scattered, dense pulmonary calcification.
CMV pneumonia is commonly seen as a mixed nodular interstitial pattern with ill-defined patchy lung infiltration. The patchy filling is caused pathologically by hemorrhage, neutrophilic and fibrinous exudates, and hyaline membrane formation (Fig. 7.2.22).
Chronic pneumonia can lead to fibrosis, traction bronchiectasis, and paracicatricial emphysema (Fig. 7.2.23).
In MRSA necrotizing pneumonia, a pneumonic patch or a pulmonary mass with central cavitary lesion can be found. The lesion can be single or multifocal. The same manifestations are observed in HRCT. Differential diagnoses of cavitary lung infiltrations include lung abscess, metastases, pulmonary lymphoma, and Wegener’s granulomatosis.

Fig. 7.2.19
Anteroposterior plain chest radiograph of a bedridden patient shows right upper lobe pneumonia with bulging of the transverse fissure (arrowhead)

Fig. 7.2.20
Posteroanterior plain chest radiograph of a 5-year-old child with measles presenting with dyspnea shows ill-defined patchy pneumonia in the upper zone of the left lung (arrowhead)

Fig. 7.2.21
Posteroanterior plain chest radiograph of a patient with varicella-zoster virus (VZV) pneumonia shows diffuse micronodular interstitial lung pattern bilaterally

Fig. 7.2.22
Posteroanterior plain chest radiograph of a patient with cytomegalovirus (CMV) pneumonia after heart transplant shows mixed patchy lung infiltration with micronodular interstitial lung pattern

Fig. 7.2.23
Posteroanterior plain chest radiograph of a patient with mycoplasma pneumonia shows right upper lobe fibrosis with honeycombing due to traction bronchiectasis (arrow) and paracicatricial emphysema (arrowheads)
Further Reading
Anuradha G. Methicillin-resistant staphylococcus aureus bacteremia and pneumonia. Dis Mon. 2008;54:787–92.
Chuang YC, et al. Negative pressure pulmonary edema: report of three cases and review of the literature. Eur Arch Otorhinolaryngol. 2007;264:1113–6.
Connolly B, et al. Bronchial artery aneurysm in hyperimmunoglobulinemia E syndrome. Pediatr Radiol. 1994;24:592–3.
Corriere MD, et al. MRSA: an evolving pathogen. Dis Mon. 2008;54:751–5.
Decker CF. Pathogenesis of MRSA infection. Dis Mon. 2008;54:774–9.
Ebert MD, et al. Necrotizing pneumonia caused by community-acquired methicillin-resistant Staphylococcus aureus: an increasing cause of “mayhem in the lung”. Emerg Radiol. 2009;16:159–62.
Fujinaga S, et al. Pulmonary edema in a boy with biopsy-proven poststreptococcal glomerulonephritis without urinary abnormalities. Pediatr Nephrol. 2007;22:154–5.
Gattinoni L, et al. The role of CT-scan studies for the diagnosis and therapy of acute respiratory distress syndrome. Clin Chest Med. 2006;27:559–70.
Kawamata M, et al. Acute pulmonary edema associated with transfusion of packed red blood cells. Intensive Care Med. 1995;21:443–6.
Kim EA, et al. Viral pneumonias in adults: radiologic and pathologic findings. Radiographics. 2002;22:S137–49.
7.3 Atelectasis (Lung Collapse)
Atelectasis is a condition characterized by lung collapse, which can be subtotal (25–50 % collapse) or total (100 % collapse).
Atelectasis can result due to air resorption (resorptive atelectasis), lung compression (compression atelectasis), or loss of the surfactant in acute respiratory distress syndrome (ARDS) and hyaline membrane disease (respiratory distress syndrome) in preterm infants (microatelectases). Pulmonary atelectasis is a recognized complication of general anesthesia.
Pulmonary surfactant is secreted by pneumocytes type II, which is composed of phospholipids and proteins. The surfactant stabilizes the lung by reducing the surface tension at the air–liquid interface in the alveoli. Therefore, deficiency of pulmonary surfactant could result in collapse of the alveolar spaces.
Patients with atelectasis commonly present with dyspnea, tachypnea, cough, and pleuritic chest pain on inspiration. Hypoxemia may result from atelectasis due to reduced ventilation–perfusion equilibrium.
Types of Pulmonary Atelectases
Resorptive atelectasis can arise due to intrinsic obstruction (e.g., mucus plug) or extrinsic obstruction (e.g., hilar lymphadenopathy). Brock’s syndrome is a term used to describe right middle lobe (RML) atelectasis by enlarged hilar lymphadenopathy compressing the right main bronchi.
Passive atelectasis results when the natural tendency of lung tissue to collapse due to elastic recoil goes unstopped. This condition can be seen in atelectasis due to pneumothorax.
Compressive atelectasis is a variant of passive atelectasis and occurs when a space-occupying lesion abuts the lung causing atelectasis (e.g., massive pleural effusion).
Cicatrization atelectasis is seen with fibrosis, where the scar tissue contracts and collapses the alveoli.
Adhesion atelectasis occurs due to surfactant deficiency, which is classically seen in hyaline membrane disease in infants and ARDS and pulmonary embolism in adults.
Plate atelectasis is composed of sheets of horizontal tissue collapse, which is commonly located 1–3 cm above the diaphragm. This type is commonly seen in conditions which impede normal respiration (e.g., inflammatory conditions in the chest or abdomen).
Congenital atelectasis is seen in newborn infants due to failure to aerate the lung after pregnancy.
Round atelectasis is seen in asbestosis, and it is characterized by atelectasis of parenchymal tissues near the pleura. It is best diagnosed by CT, which will show bronchovascular marks entering the mass (comet tail sign).
Segmental atelectasis is an uncommon type of atelectasis characterized by an entire lung segment collapse. It is highly suggestive of a tumor blocking the bronchial feeding of that segment.
Signs on Chest Radiograph
Diaphragmatic elevation due to reduced lung volume.
Shift of the right horizontal fissure upward due to upper lobe collapse (Fig. 7.3.24).
RML and left lower lobe (LLL) atelectases are located behind the heart. They can be seen as dense radio-opaque triangles overlying the heart shadow (Figs. 7.3.25 and 7.3.26). They can be easily missed if the atelectasis is examined in posteroanterior view only; lateral views are advised if RML or LLL atelectases are suspected.
Left upper lobe (LUL) atelectasis is generally seen as increase in lung density on posteroanterior (PA) view. This is explained by the fact that the LUL collapses anteriorly. Lateral view is shown clearly as an anterior mediastinal radio-opaque shadow representing the collapsed lobe (Fig. 7.3.27).
Right upper lobe (RUL) atelectasis is seen as homogenous opacity located at the right upper lung zone and bounded inferiorly by the transverse fissure (Fig. 7.3.28).
Shift of the trachea and the mediastinum toward the collapse.
For spine sign, normally the lower vertebrae on lateral view are less dense than the upper vertebra. The upper vertebrae appear denser due to the arm and axilla shadow overlying them. With progressive atelectasis of the lower lobes, the lobes will move more posteromedially, making the lower vertebra appears as dense as the upper vertebrae (Fig. 7.3.26).
Golden S sign is seen when the RUL is collapsed due to hilar mass blocking the right main bronchus (e.g., in Brock’s syndrome).
Plate atelectasis is detected on radiographs as linear horizontal radio-opaque lines commonly located 1–3 cm above the diaphragm (Fig. 7.3.29).

Fig. 7.3.24
Anteroposterior plain chest radiograph of a bedridden patient shows mediastinal shift toward the left side due to collapse of the left lung

Fig. 7.3.25
Posteroanterior (a) and lateral (b) plain chest radiographs show right middle lobe (RML) atelectasis (arrowheads)

Fig. 7.3.26
Posteroanterior (a) and lateral (b) plain chest radiographs show left lower lobe (LLL) atelectasis (arrowheads). Notice that the lower thoracic vertebrae appear denser than the upper thoracic vertebrae due to the shadow of the atelectatic lobe overlying them (spine sign)

Fig. 7.3.27
Posteroanterior (a) and lateral (b) plain chest radiographs show left upper lobe (LUL) atelectasis. Notice the high-density left lung field in (a), which is explained by atelectasis of the LUL anteriorly in (b) (arrowheads)

Fig. 7.3.28
Posteroanterior plain chest radiographs show right upper lobe (RUL) atelectasis bounded inferiorly by the transverse fissure (arrowheads)

Fig. 7.3.29
Posteroanterior plain chest radiograph shows plate atelectasis as thick radio-opaque shadow at the right costophrenic angle
Further Reading
Glay J, et al. Unusual pattern of left lower lobe atelectasis. Radiology. 1981;141:331–3.
Sargent MA, et al. Atelectasis on pediatric chest CT: comparison of sedation techniques. Pediatr Radiol. 1999;29:509–13.
Tsai KL, et al. Pulmonary atelectasis: a frequent alternative diagnosis in patients undergoing CT-PA for suspected pulmonary embolism. Emerg Radiol. 2004;10:282–6.
Westcott JL, et al. Plate atelectasis. Radiology. 1985;155:1–9.
Zhao Y, et al. Atelectasis: an unusual and late complication of lung transplant. Clin Transplant. 2002;16:233–9.
7.4 Sarcoidosis
Sarcoidosis, also known as Boeck’s sarcoid, is a multisystemic granulomatous disorder characterized by the formation of multiple epithelioid granulomas within more than one system. Sarcoidosis belongs to a large family of granulomatous disorders, which includes tuberculosis, leprosy, Langerhans cell histiocytosis, and more. All members of the granulomatous disease are characterized by the formation of granulomas within the body system.
Granuloma is a specific kind of chronic inflammation, and it is a term used to describe a nodular chronic inflammation that occurs in foci (granules), with collection of macrophages called epithelioid cells. Epithelioid cells are macrophages with abundant cytoplasm that is similar to the cytoplasm of epithelial cells. When multiple epithelioid cells fuse together, they form a bigger macrophage known as giant cell. Epithelioid cells define granulomatous inflammation. On histological specimens, granulomas show endarteritis obliterans, fibrosis, and chronic inflammatory cells (epithelioid cells). Granuloma can be due to an infection (e.g., tuberculosis) or due to an inorganic foreign body (e.g., silicosis).
Langerhans cells are characteristically found within the sarcoid granuloma, which develops by the fusion of epithelioid cells, resulting in a modified macrophage with nuclei arranged in an arc-like pattern. Langerhans cells secrete lysozyme, collagenase, calcitriol, angiotensin-converting enzyme (ACE), and varied cytokines.
No body tissue is spared from sarcoidosis. There are two forms of the disease, an acute form and chronic form. Acute sarcoidosis responds well to steroids with frequent spontaneous recovery. Moreover, it is characterized by serum elevation of ACE in two thirds of patients and abnormal calcium metabolism (high serum calcium levels). In contrast, the chronic sarcoidosis is persistent, and the serum levels of calcium and ACE are often normal.
Sarcoidosis is often seen between 20 and 40 years of age. However, juvenile form (pediatric sarcoidosis) with a smaller age peak at 13–15 years has been reported to occur rarely.
Sarcoidosis manifestations are seen in almost any part of the body. The definite diagnosis is based on histopathology examination. Radiological investigations play an important role in monitoring the therapy and the disease progression.
Pulmonary Sarcoidosis
The pulmonary system is involved in up to 90 % of patients with sarcoidosis. Patients with sarcoidosis are classically young females presenting with nonspecific symptoms of a systemic disease (e.g., malaise). In pulmonary sarcoidosis, dyspnea and cough are common, whereas hemoptysis (coughing blood) is rare.
Sarcoidosis Has Five Radiological Grades on Plain Chest Radiograph
Grade 0: Normal chest radiograph.
Grade 1: There are clear lung fields with bilateral hilar lymphadenopathy (85 % of cases). It is usually identified by accident, and the patient is asymptomatic (Fig. 7.4.30).

Fig. 7.4.30
Posteroanterior chest radiograph of a female patient with grade 2 pulmonary sarcoidosis shows bilateral hilar lymphadenopathy (arrowheads) with clear lung fields
Grade 2: There is reticulonodular interstitial pattern with hilar lymphadenopathy. The areas affected are usually located in the upper lobes.
Grade 3: There is reticulonodular interstitial pattern without hilar lymphadenopathy (Fig. 7.4.31).

Fig. 7.4.31
Posteroanterior chest radiograph of a patient with grade 3 pulmonary sarcoidosis shows bilateral diffuse reticular interstitial pattern due to lung fibrosis
Grade 4: There is pulmonary parenchymal scarring and fibrosis (Fig. 7.4.32).

Fig. 7.4.32
Posteroanterior chest radiograph of a patient with chronic grade 4 pulmonary sarcoidosis shows bilateral lung fibrosis distorting the heart silhouette (shaggy heart appearance)
Signs on HRCT
Sarcoid granulomas are typically distributed along the lymphatic vessels within the interstitium. Due to this fact, miliary nodules plus linear thickening of the interlobar septa can be found in a similar fashion to the interstitial disease seen in lymphangitis carcinomatosis and lymphoproliferative diseases.
Bilateral hilar lymphadenopathy observed in grade 2 and 4. Punctuate, stippled, or egg shell calcification patterns may be seen.
Occasionally, multiple granulomas may aggregate to form a mass-like nodule within the lungs that mimics metastasis (Fig. 7.4.33). Lung nodule is a lesion <3 cm in diameter, whereas lung mass is a lesion >3 cm in diameter.
Necrotizing sarcoid granulomatosis is a rare variant of sarcoid characterized by the formation of cavitating granulomas.
Hilar lymphadenopathy with eggshell calcification and bilateral upper lobe fibrosis are typical findings in pulmonary silicosis. Sarcoidosis may mimic silicosis when it produces the same set of radiographic manifestations on plain chest radiograph.

Fig. 7.4.33
Axial thoracic lung-window HRCT illustration demonstrates a pulmonary mass that is composed of multiple aggregated sarcoid granulomas. Although it is a difficult diagnosis to confirm without biopsy, the presence of air bronchogram or areas of normal tissue lines within the mass (arrowhead) can differentiate this rare lesion from bronchogenic carcinoma
Hepatic, Splenic, and Gastric Sarcoidosis
Hepatic sarcoidosis is seen in 5–15 % of patients with high ACE levels (acute disease). There are multiple hepatic granulomas that can be easily mistaken on CT and MRI for metastasis or lymphoma. Simultaneous involvement of the spleen favors the diagnosis of sarcoidosis and lymphoma.
Splenic sarcoidosis classically affects the white bulb and the arterial circulation. The spleen is affected in 5–14 % of patients with sarcoidosis. Patients may suffer from symptoms of hypersplenism, anemia, thrombocytopenia, and leukopenia.
Gastric sarcoidosis is the most common feature of gastrointestinal involvement of sarcoidosis. It often involves the antrum. Massive retroperitoneal lymphadenopathy may be rarely encountered in sarcoidosis.
Signs on CT
Hepatic sarcoidosis is seen as multiple hypodense lesions with irregular shapes on liver contrast-enhanced images.
Splenic sarcoidosis is seen on contrast-enhanced images as multiple, irregularly diffuse, hypodense lesions within the spleen representing granulomas (Fig. 7.4.34). Hepatic lesions may be noticed in the same scan (50 % of cases). The same lesions are seen hypoechoic on US and hypointense on T1W and T2W images on MRI compared to the background.
Gastric sarcoidosis features range from ulceration mimicking peptic ulcer to mucosal thickening mimicking Menetrier disease. Diagnosis requires endoscopic biopsy to confirm the epithelioid granuloma.

Fig. 7.4.34
Abdominal nonenhanced CT illustration demonstrates multiple hypodense lesions within the spleen representing splenic sarcoid granulomas
Dermatological Sarcoidosis
Skin lesions are seen in up to 25 % of patients with sarcoidosis. Skin lesions in sarcoidosis are divided into reactive and specific lesions. Reactive sarcoidosis skin lesions do not contain granuloma formation histologically (e.g., erythema nodosum). In contrast, specific sarcoidosis skin lesions are characterized by noncaseating granuloma formation (e.g., Darier–Roussy nodules).
In the acute reactive sarcoidosis, erythema nodosum is the most common finding, and it is seen as multiple patchy red lesions found over the shin, often in a bilateral fashion. Systemic manifestations like fever, malaise, and polyarthralgia occur in about 50 % of patients with erythema nodosum.
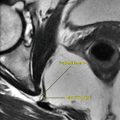
The chronic reactive sarcoidosis, on the other hand, is characterized by a specific lesion called “lupus pernio.” Lupus pernio is a specific skin lesion in sarcoidosis characterized by dusky-red plaques formation on the nose, ears, lips, and face. Lupus pernio is classically seen in women with chronic sarcoidosis and extensive pulmonary infiltration, anterior uveitis, and bone lesions. The nose lesion is typically red to purple in color and seen on the tip of the nose, causing bulbous appearance (Fig. 7.4.35). The nose lesion infiltrates the mucosa and may destroy the underlying nasal bone.


Fig. 7.4.35
An illustration demonstrates lupus pernio on the ala of the nose
In black patients, maculopapular eruptions are the most common skin manifestations of sarcoidosis. Darier–Roussy nodules are small painless subcutaneous nodules that arise within the dermis and the epidermis. They represent noncaseating granulomas.
Differential Diagnoses and Related Diseases
Löfgren syndrome is a disease characterized by the combination of arthralgia, bilateral hilar lymphadenopathy, and erythema nodosum in a patient with sarcoidosis.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree