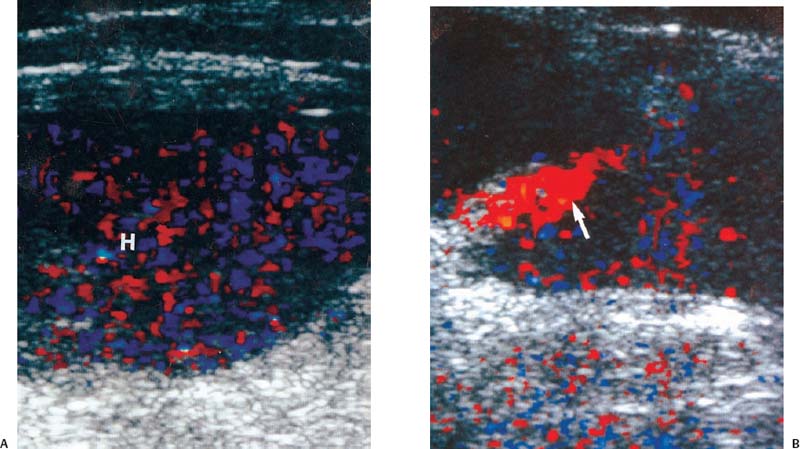
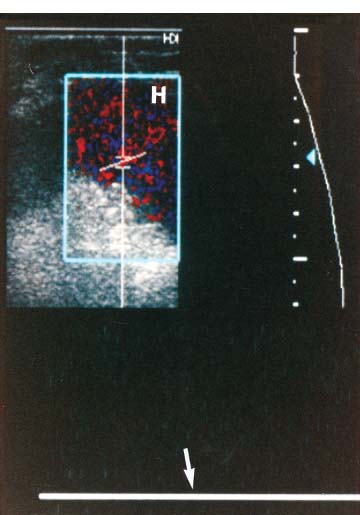
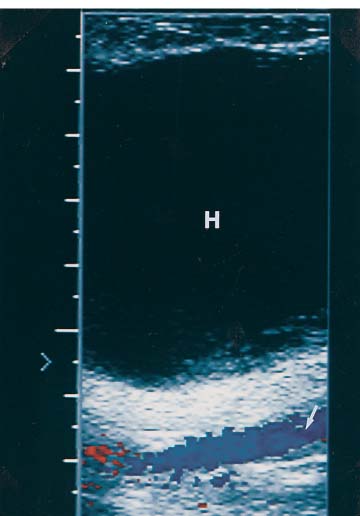
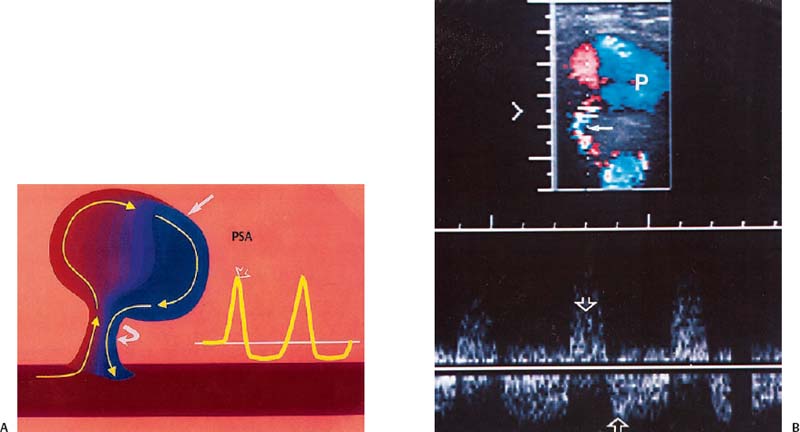
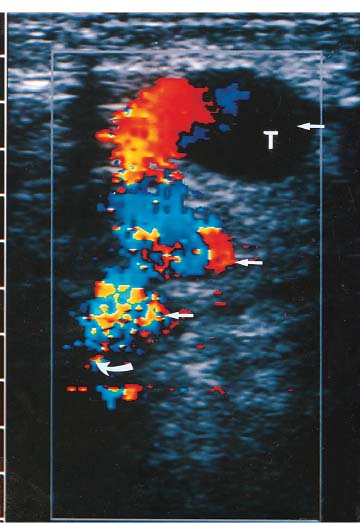
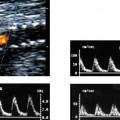
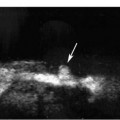
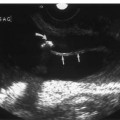
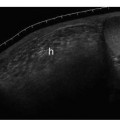
3 Pulsatile Groin Mass in the Postcatheterization Patient Percutaneous catheterization of femoral vessels is a rapidly increasing method for performing diagnostic and therapeutic procedures as an alternative to more invasive surgical interventions. As the number of procedures and their complexity, utilizing larger vascular sheaths, lytic agents, and anticoagulants, increases, the number of postcatheterization groin complications has also grown. Although the majority of groin interventional procedures are followed by little more than a relatively small bruise, significant complications including large hematomas, pseudoaneurysms (PSAs), and arteriovenous fistulas (AVFs) can occur. The frequency of postcatheterization complications varies depending upon the nature of the intervention, whether diagnostic or therapeutic, and relative meticulousness of postsheath removal techniques utilized. Complication frequencies reportedly range from less than 1% for diagnostic catheterizations, up to 9% for coronary angioplasty, and as high as 16% following placement of an intracoronary stent.1 Color Doppler ultra-sound is now the preferred technique for evaluating potential groin complications related to femoral artery catheterization.2–5 The color Doppler findings characteristic of these complications will be presented, including unusual complications, potential diagnostic pitfalls, and an approach to ultrasound-guided compression pseudo-aneurysm repair. The majority of patients referred for a groin ultrasound examination after femoral artery catheterization have a palpable mass. These masses may be pulsatile and/or associated with a new bruit or palpable thrill. Color Doppler ultrasound provides a rapid, relatively pain free, portable imaging technique that can readily discern the cause of these auscultatory and palpable abnormalities. The examination is usually performed using a 5 MHz linear array transducer; however, a 7.5 MHz may be used for thin patients or for superficial lesions, whereas for obese patients or those with large hematomas overlying the vascular structures, a 3.5 MHz transducer may be required. The examination consists of color and pulsed Doppler evaluation of the groin vessels beginning at the region of the inguinal ligament above the puncture site, then at and below the puncture site. Color Doppler should be used to confirm the patency of the femoral artery and vein and search the extravascular soft tissues for evidence of large hematomas, PSAs, or AVFs. The color Doppler gain threshold and flow detection parameters should be adjusted so that color fills the artery and vein, but does not “bleed over” into the adjacent soft tissues. It may be necessary to decrease the gain or increase the pulse repetition frequency (PRF) to visualize the artery and vein if a significant color Doppler bruit secondary to an AVF is present. Similarly, gain and threshold adjustments may be necessary to eliminate color noise artificially written into soft tissues, such as hematomas, which may be confused with PSAs (Fig. 3–1A,B). A complete ultrasound evaluation of the postcatheterization groin rarely takes more than 15 to 20 minutes. Alternative imaging tests are rarely needed; however, occasionally computed tomography (CT) will be obtained to evaluate the extent of a retroperitoneal hematoma related to postcatheterization complications, and arteriography may occasionally be needed before surgical or percutaneous interventions. Virtually all patients who have undergone a groin catheterization have a small, localized hematoma or bruise. However, when the ecchymosis is extensive or there is a palpable mass, which may be pulsatile, patients are usually referred for color Doppler sonography. Localized hematomas present as discrete hypo- to anechoic masses that do not contain blood flow. Color Doppler noise may appear in a hematoma if gain settings are not optimized; pulsed Doppler interrogation will show the absence of a characteristic signal (Fig. 3–2). Large hematomas may compress and displace femoral vessels posteriorly such that they are beyond the penetration range of higher-frequency range transducers (Fig. 3–3). A localized hematoma and a thrombosed PSA are indistinguishable in appearance. Occasionally a hypoechoic tract leading from the vessel to the thrombosed PSA will be seen. Although hematomas and thrombosed PSAs are both avascular, inflammatory hypervascularity may be seen surrounding hematomas. Additionally, actively bleeding jets may be seen into a rapidly expanding hematoma (Fig. 3–1B). Many clinically obvious hematomas are difficult to recognize on ultrasound because the hemorrhage has infiltrated diffusely into the soft tissues of the thigh or retroperitoneum, resulting in diffuse distortion of architecture and often suboptimal ultrasound penetration, but no discrete mass. Sonography is relatively insensitive for the evaluation of pelvic side wall or retroperitoneal hematomas. If a patient experiences a significant hematocrit drop or severe back pain following catheterization, CT is usually indicated as a means of evaluating the presence and extent of a retroperitoneal hematoma. Figure 3–1 (A) A large right thigh hematoma (H) is filled with red and blue color Doppler “noise” related to transmitted motion created by intermittent venous leakage into the large mass. (B) The area of the intermittent vascular leak (arrow) is seen. The area of the low-velocity venous leak vanished by the end of the examination. Figure 3–2 Pulsed Doppler analysis of color Doppler noise transmitted into a soft tissue hematoma (H) shows absence of flow (arrow). Figure 3–3 Large-groin hematoma (H) displaces the common femoral vein (arrow) posteriorly and compresses it. Figure 3–4 (A) Diagrammatic representation of a pseudoaneurysm demonstrates the characteristic “yin-yang” color Doppler pattern within the pseudoaneurysm (arrow) and the characteristic to-and-fro waveform (open arrow) obtained from the region of the pseudoaneurysm neck (curved arrow). (B) Duplex color Doppler image of a pseudoaneurysm (P). Note the characteristic red and blue flow pattern within the pseudoaneurysm. The pulsed Doppler waveform obtained from the neck (arrow) demonstrates the characteristic to-and-fro (open arrows) waveform. Following femoral arterial sheath removal, the artery is manually compressed to assure hemostasis at the puncture site. Typically, 15 minutes of manual compression are required following diagnostic procedures; however, therapeutic procedures may require longer periods of compression, particularly if anticoagulation is continued. A recent study showed that the incidence of PSA development following arterial catheterization was related to the type of intervention, with therapeutic procedures having a higher incidence of PSAs.5 However, the greatest risk factor for developing a PSA, regardless of the type of intervention, was too brief a period of manual compression following sheath removal. If the arterial wall defect fails to seal, then pulsatile blood jets from the artery into the adjacent perivascular soft tissues. This hematoma connects with the artery via a neck or tract and is largely contained within the soft tissues. Although these contained hematomas are referred to as PSAs, they do not have the characteristic fibrous wall of a true PSA; thus some feel it is more accurate to refer to these masses as pulsatile hematomas.2 Color Doppler examination readily distinguishes PSAs from other pulsatile masses in the postcatheterization groin. Sonographic features of a PSA include the detection of a vascular mass connected to the artery by a neck or tract. During systole there is antegrade flow into the PSA through the neck (Fig. 3–4A,B). During diastole, the increased pressure in the PSA, vis-à-vis the underlying artery results in a retrograde flow out of the PSA through the neck and back into the artery. This results in a characteristic “to-and-fro” pulsed Doppler waveform in the PSA neck and produces a characteristic “yin-yang” swirling color flow pattern within the body of the PSA. Some PSAs may be multilobed, producing a “string-of-beads” appearance (Fig. 3–5). These probably result from more extensive dissection of blood along fascial planes. Although two separate PSAs can occur following multiple punctures, multilobulated PSAs usually arise from a single puncture and interconnect between the artery and the multiple lobes. PSAs may also coexist with AVFs. In such cases, the Doppler features of either or both of the complications may be seen (Fig. 3–6A–C). These patients may present with a bruit and a pulsatile mass. Some patients may demonstrate a linear track of blood flow that follows the expected track of the needle or sheath from the artery toward the actual puncture site.6 These tracks may represent an abortive form of a PSA. However, these are very narrow, with varying waveforms not characteristic for a PSA. These tracks do not seem to be clinically important, nor do they appear to require further imaging or treatment if they are observed as isolated findings. Figure 3–5 Trilobed pseudoaneurysm. The three lobes of the pseudoaneurysm (arrows) are seen arising from a single neck (curved arrow). Thrombus (T) is seen obliterating part of the lumen of the most superficial lobe. Complications of PSAs including pain, infection, and compressive neuropathy, and most critically, rupture, can occur. Fortunately, this dire consequence is rare, but because this complication has such a potentially disastrous outcome, PSAs have been considered surgical emergencies. Recent studies have shown that PSAs are more benign and self-limited complications than previously thought.7–9 Indeed, the majority of PSAs reported in many series have spontaneously thrombosed, suggesting that close monitoring and no further treatment may be sufficient. The majority of PSAs thrombose spontaneously within a week, and if imaged serially will demonstrate progressive thrombus of the PSA. PSAs with relatively small volumes of pulsatile flow and those with long necks are more likely to thrombose than will those with larger-flow volumes and short or wide necks. Ultrasound-guided compression therapy has been enthusiastically welcomed as an alternative to surgery.10–12 This time-consuming, relatively noninvasive procedure obviates the need for surgical groin incisions, which tend to heal poorly in these patients. Another attractive aspect of this procedure is that there is no need for the expense of operating room time. Although this procedure is less invasive, it is potentially painful, and intravenous sedation is frequently needed. The procedure consists of obtaining informed consent and administering pain medications and sedation as needed. The ultrasound transducer is then used to locate the PSA neck. Vigorous manual compression is applied directly to the PSA neck to determine if blood flow into the PSA can be completely obliterated (Fig. 3–7A–C
Diagnostic Evaluation
Ultrasound Imaging
Hematomas
Pseudoaneurysms
Ultrasound-Guided Compression
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree