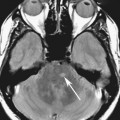
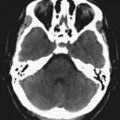
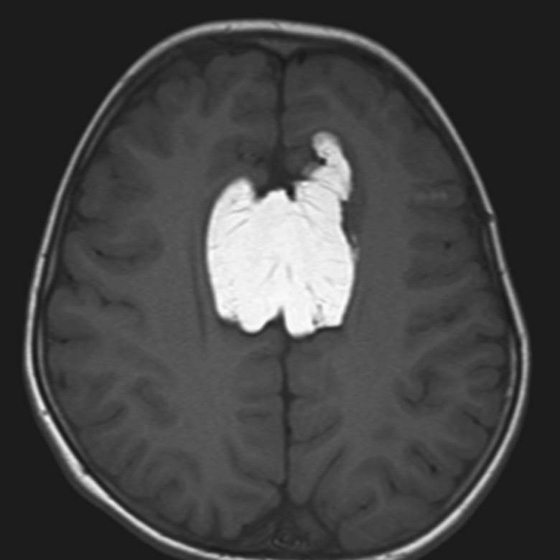
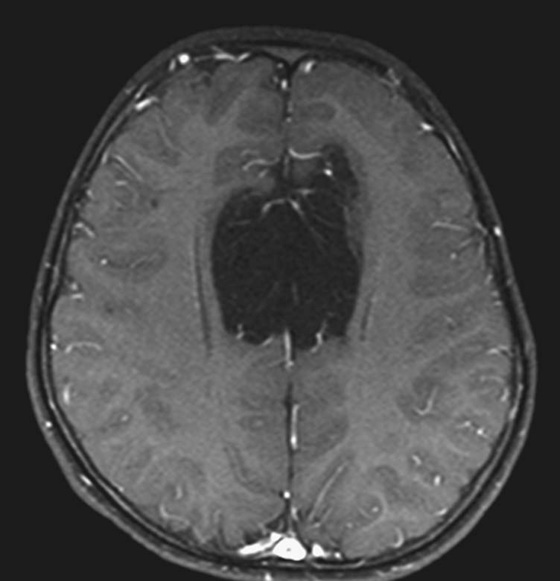
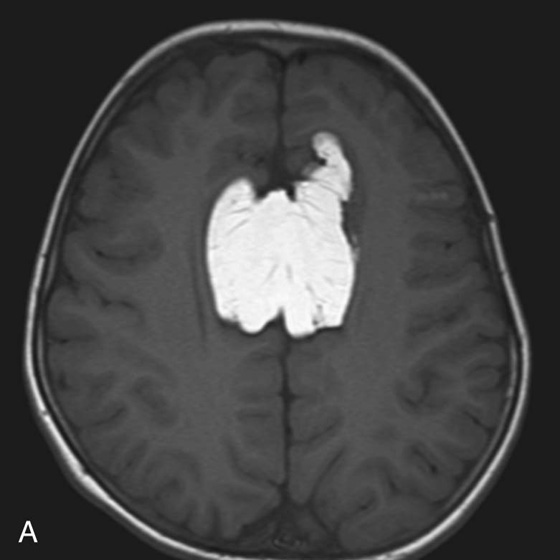
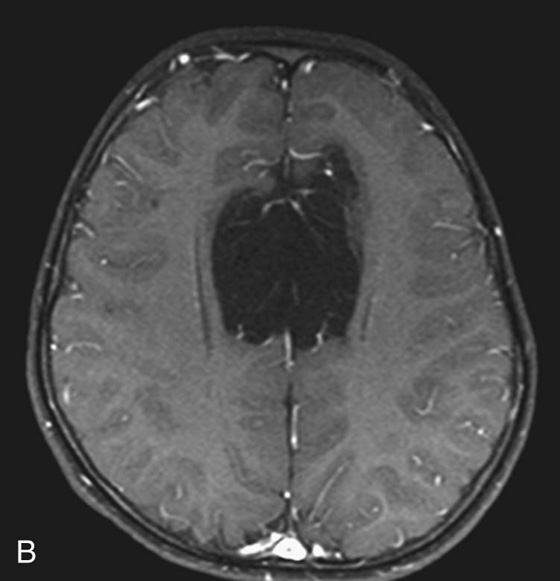
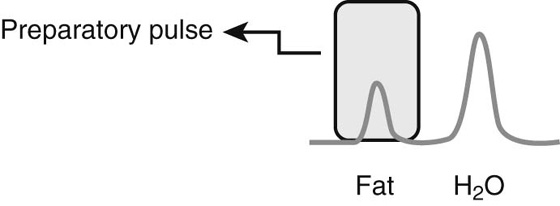
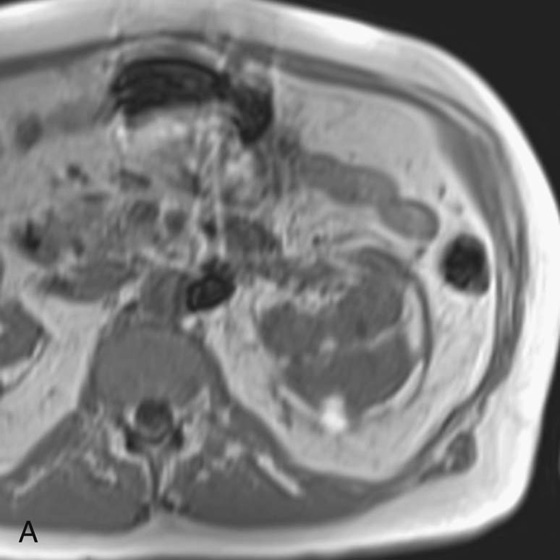
Chapter 5 Phil B. Hoang, Matthew P. Lungren, Allen W. Song, and Elmar M. Merkle 1. Name some common causes of high signal on a T1W image. 2. What are the two most common techniques to suppress fat signal? 3. Which of these techniques specifically suppresses signal from fat? 4. What is the diagnosis? FIGURE 1A. Axial T1-weighted (T1W) image demonstrates the mass’s interhemispheric position; thin, linear hypointense septa are noted. FIGURE 1B. Axial postcontrast T1W image with fat saturation. Near-complete signal loss of the mass has occurred, with thin linear internal septations now evident. 1. Fat, subacute hemorrhage, proteinaceous fluid and melanin can all cause high T1 signal. 2. Frequency-selective fat saturation and short tau inversion recovery are the two most commonly used fat suppression techniques in MRI. 3. Frequency-selective fat saturation specifically suppresses signal from fat. 4. The diagnosis is a lipoma of the corpus callosum. The common link between inversion recovery, spatial-selective presaturation, frequency-selective, and magnetization transfer sequences is the use of a radiofrequency (RF) pulse administered prior to the excitation pulse. This is referred to as the preparatory pulse. This chapter covers frequency-selective and spatial-selective presaturation pulses, while Chapter 6 reviews inversion recovery. Frequency-selective saturation is a widely used and versatile technique. It is based on the assumption that a hydrogen proton resonates at a certain frequency based on its unique molecular environment. Protons in water and fat resonate at different frequencies when under the same external magnetic field because of the micromolecular environments unique to each tissue. These differences increase with increases in magnetic field strength. For example, protons in fat precess 74 Hz slower than protons in free water at 0.5T, 220 Hz slower at 1.5T, and 448 Hz slower at 3T. Frequency-selective saturation takes advantage of these differences in precessional frequencies, which allows signal suppression of either fat or water (Fig. 1). Once the target protons are saturated (Fig. 2), they are dephased when a spoiler gradient is applied (Fig. 3). This spoiling of the protons’ transverse magnetization suppresses their ability to produce signal. The excitation pulse is sent soon after. As mentioned before, water or fat signal may be suppressed if the appropriate RF saturation pulse is used. Because the frequency-selective saturation pulse is broadcast at a narrow-range RF to specifically affect the target protons, the quality of signal suppression depends on a uniform magnetic field. The presence of magnetic field inhomogeneities typically results in poor signal suppression and, at times, inadvertent signal suppression of nontarget tissues. Also, because fat has a short T1 relaxation time, it recovers a measure of its net longitudinal magnetization during the time between the preparatory and excitation pulses. This may require multiple fat saturation pulses to obtain the preferred signal loss, which contributes to RF energy deposition in the patient. Frequency-selective techniques are typically not used at lower magnetic field strengths (< 1.0 Tesla) as there is an excessive overlap of resonant frequencies between water and fat protons; this leads to not only incomplete saturation of the desired protons, but also saturation of nontarget protons. Another setting where a preparatory pulse is administered to affect tissue contrast is magnetization transfer (MT). MT refers to the transfer of longitudinal magnetization from restricted water protons to free water protons. This technique is based on the concept that restricted water protons are generally bound to macromolecules (e.g., proteins or lipids), and thereby subject to greater local field inhomogeneities. Free water protons, in contrast, exist in a comparatively uniform magnetic field environment. The different environments lead to different ranges of resonance frequencies of the water protons; free water will have a narrow resonance frequency range near 63 MHz (1.5T), while the range of resonance frequencies in bound water protons will be much broader owing to the inherent field inhomogeneities in their environment. Therefore, an “off-resonance” preparatory pulse will preferentially saturate bound water protons. What allows for variable soft tissue contrast, however, is the phenomenon by which magnetization is then transferred from bound water protons to available adjacent free water protons, leading to a saturation effect. The result is reduced signal from the free water protons in tissues where the magnetization transfer phenomenon is prevalent. Because the extent of signal decay depends on the exchange rate between free and bound water protons, MT can be used to provide an alternative contrast method to complement T1, T2, and proton density methods. The most accepted application is in magnetic resonance angiography, where MT markedly suppresses background tissue signal while leaving flowing blood unaffected. Magnetization transfer is also believed to be a nonspecific indicator of the structural integrity of the tissues, and has found promising applicability in highlighting specific tissue abnormalities in the brain (e.g., demyelination in multiple sclerosis).6 Spatial-selective presaturation is a technique used to suppress signal arising from regions of the body outside the imaging area of interest. A common clinical setting where spatial-selective presaturation is used is lumbar spine imaging, where reduction of phase-related motion artifact arising from peristalsing bowel is achieved with application of a saturation band placed over the abdomen.
Preparatory Pulses
CASE 1
ANSWERS
CASE 1
Preparatory Pulses Part I: Saturation Pulses
CASES 2, 3, AND 4: COMPANION CASES
Case 2
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Pulses