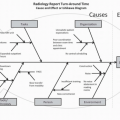
a government-approved, evidence-based appropriate-use criteria through a CDSS prior to ordering examinations.5,6 Thus far, the published data from other radiology subspecialties that introduced CDSSs in their clinical practice have identified a significant reduction in inappropriate imaging.7,8,9,10,11 Although advanced imaging studies constitute a smaller proportion of a breast imaging practice volumes, the use of CDSS that incorporates ACR AC for common clinical and imaging conditions of the breast can be extended to the ordering process for the entire spectrum of breast imaging examinations.
Initial certification any time new mammography equipment is installed or equipment replaced.
Yearly renewal for the already certified facilities and equipment
Submitting an application for certification (initial or renewal) that includes:
The name and qualifications of the designated lead interpreting radiologist who is ultimately responsible that the quality control (QC) tests and measures have been met
The name and qualifications of the designated lead mammography technologist who oversees the performance of the QC tests
The name and qualifications of the designated lead physicist who performs the required annual tests and produces the annual report
The information on each mammography unit used for clinical imaging at that location (vendor, make, and model)
Types and number of mammographic examinations performed (screening versus diagnostic) in the last 12 months
The names and qualifications (educational background, training, and professional experience) of all the radiologists interpreting the mammographic examinations
The names and qualifications of all the technologists performing the mammography examinations
Application fees for the facility and each mammography unit
A physical site visit by a designated state or FDA mammography inspector. During this site visit, the inspector will review:
Records of completing the recommended QC tests for each mammography unit in that facility for the past 12 months
Records of initial certification and maintenance of certification requirements for each radiologist interpreting mammographic examinations at that facility
Records of initial certification and maintenance of certification requirements for each mammography technologist acquiring mammographic examinations
Records of the medical outcomes audit
There are some general QC requirements that are common between the different imaging acquisition techniques (screen-film versus digital imaging) and between different commercially available manufacturers, while other requirements or timing of tests will be specific to individual systems (Table 8.1).
Table 8.1 EXAMPLE OF MAMMOGRAPHY QC TESTS FOR ONE OF THE COMMERCIALLY AVAILABLE DIGITAL IMAGING SYSTEMS.
Category
Location of Test
Name of QC Test or Procedure
Description/Objective
Frequency
Tools
Image viewing
Reading room
Monitor cleaning/viewing conditions
To assure cleanliness of the monitors from dust, finger print, or particles
Daily
Specific cleaning instructions per manufacturer
Diagnostic review workstations QC—display calibration check
To assure consistency and calibration of brightness and contrast of the diagnostic workstation
Daily (or monthly depending on vendor)
Society of Motion Picture and Television Engineers (SMPTE) pattern
Display phantom
To assure quality and consistency of mammographic image
Weekly
ACR phantom
Image printing
Tech work area
DICOM printer QC
To assure consistency of DICOM printer performance
Weekly; after PM service; after software change
SMPTE pattern printed from imaging equipment (for density measurement and artifacts); densitometer
Image acquisition
Examination room
Detector flat field calibration
To assure proper system calibration
Weekly
Flat field phantom
System artifact
To assure lack of artifact during image acquisition
Weekly
Flat field phantom analyzed on the diagnostic review workstation
SNR and contrast-to-noise ratio (CNR)
To assure consistency of the image receptor
Weekly
ACR phantom analyzed on the acquisition workstation
Compression thickness indicator
To assure the indicated compression thickness is within tolerance
Biweekly (every 2 wk)
Visual check of equipment
To assure all equipment in the examination room and system are mechanically stable (system indicator lights, displays, mechanical locks, cones, collimators, hand switch, angulation indicator, control booth)
Monthly
Repeat analysis
To assure technologists are adequately trained on patient positioning
Quarterly
Checking records for percentage of examinations requiring additional views; should be between 2% and 5%
Compression force
To assure the system can provide adequate compression through manual or power-assisted mode and that compression is controlled
Semiannually
Bathroom scale, towels
System analysis
Routine service PM by the manufacturer
Semiannually
The vendor’s service team
Manufacturers will routinely provide technologists training on the specifics of QC tests, with the installation or upgrade of their equipment. Additionally, they will provide detailed QC manuals and checklists that can guide the technologists during routine QC tests.
The role of the lead mammography technologist is to perform mammography or identify key technologists who will be delegated the specific QC tests and checklists as well as to ensure that all the QC
tests are performed according to the timing and technique specified by individual manufacturers and are well documented. Prior studies have estimated that the time involvement in maintaining the QC tests is approximately 160 hours per year.17
The role of the lead interpreting physician is to work in close collaboration with lead mammography technologist in oversight of the QC process and documentation, in creation of QA policies, procedures, and workflow strategies to fulfill the MQSA regulations (Table 8.2), and to identify areas for workflow improvement. Additionally, one of the key roles of the lead interpreting radiologists is to closely review the medical outcomes audit for the entire practice and for individual radiologists and to compare these metrics to national benchmarks. This is crucial for identifying trends or outlying performers and facilitating practice improvement measures. This will further be discussed in the Medical Outcomes Audit section.
Table 8.2 EXAMPLES OF DIFFERENT STANDARD OPERATING POLICIES (SOP) AND PROCEDURES TO MEET REQUIREMENTS FOR MQSA ACCREDITATION FOR MAMMOGRAPHY. | |
|---|---|
|
Table 8.3 MAMMOGRAPHY PROGRAM: MQSA AND ACR REQUIREMENTS FOR INITIAL AND CONTINUED CERTIFICATION FOR INTERPRETING RADIOLOGISTS. | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
A new unit is installed
A previously accredited unit moved from one location to another
Major repair or upgrades
This constitutes part of the MEE and is included with certification paperwork and kept with the QC documents (www.fda.gov/RegulatoryInformation/Guidances/ucm094405.htm).
Table 8.4 MAMMOGRAPHY PROGRAM: MQSA AND ACR REQUIREMENTS FOR INITIAL AND CONTINUED CERTIFICATION FOR TECHNOLOGISTS. | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Table 8.5 MAMMOGRAPHY PROGRAM: MQSA AND ACR REQUIREMENTS FOR INITIAL AND CONTINUED CERTIFICATION FOR PHYSICISTS. | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Facilities must ensure that final mammography reports are sent to the referring physicians as well as a summary of these results sent to the patient in lay terms as soon as possible, no longer than 30 days.
Facilities must keep patients’ prior mammograms as part of their permanent medical records somewhere between 5 and 10 years.
The majority of mammography facilities across the United States will need to be accredited by the ACR. The exceptions are the facilities in Texas, Arkansas, or Iowa, which can be accredited by their respective state departments of HHS.
The ACR certification process has to be initiated for each clinic or imaging center that performs mammography, even when belonging to the same organization or radiology group, and it entails the certification of each imaging unit within that location.
The ACR accreditation for mammography adheres to the same requirements as the FDA certification. In addition, it assures the quality of images acquired at each facility by reviewing an example of the facility’s clinical images, which are submitted at the time of certification.
Although onsite visit is not mandatory for ACR accreditation, the ACR will conduct onsite survey to random sample of mammography facilities across the United States. If a facility is chosen for a random site visit, it will be notified in advance. The survey team will include the ACR radiologist, medical physicist, and ACR staff technologist, and items that will be reviewed during their visit include:
Documentation of personnel qualifications
Documentation of the QA program
Documentation of the policies and procedures
Mammography images and reports from clinical cases
Initial certification any time new mammography equipment is installed or equipment replaced.
Renewal process for the already certified equipment. This is conducted on a 3-year accreditation cycle. The ACR will send a notification of renewal 8 months prior to the expiration of the accreditation cycle.
Basic facility information, including detailed equipment information and personnel information
The detailed medical physicist’s MEE; summary report detailing that all the equipment meets FDA specifications and have passed all required FDA QA tests, as detailed in the FDA Certification section.
Certification fees
Personnel information and qualifications (physicians, technologists, physicist)
Facility policies and QA procedures, including reporting mechanism
Medical outcomes audit
QC results
Clinical images
of accurate data accrual, a second step of calculations of derived data, a third step of reviewing and analyzing the outcomes data and comparing it to national benchmarks, and lastly, the fourth step of introducing practice improvement measures (Fig. 8.4). The initial three steps can then be repeated for assessing the impact of the introduced practice improvement measure.
 FIG. 8.3 • Digital image of the ACR phantom: technically adequate imaging system with at least four (4) fibers, three (3) speck groups, and three (3) masses are identified. |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree