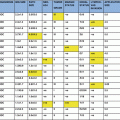
Matched pair analysis (I)
EBRT + boost (III)
Mannheim TARGIT-A QoL trial (II)
IORT
EBRT
IORT-EBRT
IORT-boost
EBRT-boost
Mean (SD)
Mean (SD)
Mean (SD)
Mean (SD)
Mean (SD)
General pain
I
23.9 (24.5)
27.5 (34.7)
42.8 (32.9)
36.5 (35.0)
26.7 (29.8)
II
21.3 (33.2)
40.9 (32.3)
43.8 (32.1)
Breast symptoms
I
8.6 (12.3)
19.2 (23.8)
26.1 (27.6)
27.0 (24.4)
21.9 (24.1)
II
7.0 (14.0)
19.0 (20.0)
29.7 (22.8)
Arm symptoms
I
22.2 (25.9)
21.7 (27.7)
37.2 (36.5)
32.5 (28.7)
27.6 (29.1)
II
15.1 (22.2)
32.8 (28.6)
32.6 (25.8)
Role functioning
I
71.7 (30.7)
73.2 (25.7)
65.9 (26.8)
63.7 (31.8)
70.8 (30.8)
II
78.7 (35.2)
60.5 (29.5)
65.6 (29.5)
Global health status
I
70.3 (23.0)
70.3 (23.9)
57.6 (20.7)
59.6 (21.7)
72.0 (20.2)
II
63.3 (24.2)
52.4 (22.1)
60.9 (19.9)
Medical co-morbidities ≥2
I
56 %
39 %
56 %
54 %
17 %
II
56 %
80 %
69 %
ALND
I
74 %
65 %
100 %
61 %
79 %
II
16 %
30 %
25 %
9.1.3 Conclusion
To summarise, in contrast to toxicity reports, very few studies have investigated QoL after IORT. The available research is limited by its focus on breast cancer patients. Patients with early breast cancer treated with breast-conserving surgery and IORT +/− EBRT present with excellent QoL scores and few symptoms compared with patients receiving EBRT without a boost. In the randomised setting, several general health-related and disease-specific QoL domains (restrictions in daily activities, i.e. role functioning, general pain, breast and arm symptoms) are superior after IORT than after EBRT. Non-randomised comparisons show equivalent parameters in the IORT-EBRT group and the IORT-boost or EBRT-boost control group.
9.2 Late Radiation Toxicity (E. Sperk)
Understanding of the indications for and use of IORT has grown as increasing numbers of patients have been treated. In 2004 the level of evidence was scored IV in regard to the postoperative complications seen in 25 patients treated with IORT using INTRABEAM (Cuncins-Hearn et al. 2004). During the past 10 years, the TARGIT-A trial has recruited a total of 3,451 patients, randomised consistently into the experimental arm with IORT (n = 1,721) or the standard arm with EBRT (n = 1,730). The first results, presented in 2010, showed non-inferiority of IORT as a sole treatment in early stage breast cancer within the scope of a randomised, international, multi-centre phase 3 trial (Vaidya et al. 2010).
Generally, late toxicity is classified as toxicity occurring 90 days or more after treatment. Different scores exist to assess late toxicity, and all of them have specific advantages and drawbacks. Some are detailed while others are less precise in definition of the differences from 0 to I, II, III or IV. The most common toxicity scores are the CTCAE (Common Terminology Criteria of Adverse Events) defined by the EORTC (European Organisation for Research and Treatment of Cancer), the RTOG (Radiation Therapy Oncology Group) or other leading organisations. For late toxicities the LENT SOMA (Late Effects of Normal Tissue; Subjective, Objective, Management, and Analytic) scales are usually used. The LENT SOMA tables were well described in 1995 and are used for almost all entities (No authors listed 1995). With these tables it is possible to achieve a high level of objectivity, though a certain subjectivity will always remain. Hoeller et al. compared the RTOG and LENT SOMA scores in a group of breast cancer patients (n = 259). The impact of the classification system on grading late effects was evaluated. The study group concluded that LENT SOMA criteria seem to be a better tool than the RTOG scale for grading and recording late radiation toxicity because use of the LENT/SOMA criteria resulted in some upgrading of skin toxicity in comparison with the RTOG score. In contrast, fibrosis scores correlated very well (Hoeller et al. 2003).
IORT for breast cancer is used in a number of situations: (a) breast cancer recurrence during second breast-conserving surgery to avoid mastectomy, (b) as an advanced boost followed by external whole breast radiotherapy or (c) as a single treatment in early stage breast cancer. Table 9.2 gives an overview of all applications for IORT in breast cancer and the available toxicity data until December 2012.
Table 9.2
Late toxicity after IORT: an overview for all applications
Study | Year | N | F-U (months) | LR (%) | Chronic skin toxicity | Toxicity |
|---|---|---|---|---|---|---|
Pre-irradiated | ||||||
Kraus-Tiefenbacher et al. | 15 | 26 | 0 (0) | RTOG °III–IV: 0 % | ||
Keshtgar et al. | 21 | 42 | 0 (0) | No unexpected | ||
Kraus-Tiefenbacher et al. | 20 | 37 | 2 (10) | T: 5 % | Fibrosis° II–III: 45 % | |
H: 5% | ||||||
IORT as a boost | ||||||
Kraus-Tiefenbacher et al. | 73 | 25 | T: 0 % | RTOG °III–IV: 0 % | ||
Wenz et al. | 48 | 36 | T: 6 % | °0–I: 63 % | ||
H: 8 % | Fibrosis °II–III: 23 % | |||||
Wenz et al. | 155 | 34
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

| ||||