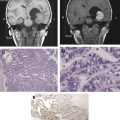
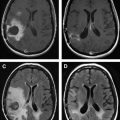
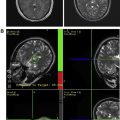
Radiation therapy remains a critical therapeutic modality in the treatment of adult brain tumors. However, its use continues to evolve depending on the histologic findings of the brain tumor. In high-grade gliomas, current trials focus on the addition of systemic agents and optimization of target delineation to improve the therapeutic ratio of radiotherapy. In low-grade gliomas, the life expectancy is much greater, and the possibility of late effects of radiotherapy have shaped contemporary trials to attempt to identify groups that benefit from radiotherapy versus the ones that may defer radiotherapy until tumor progression. With primary central nervous system lymphoma, the advent of high-dose methotrexate-based chemotherapy and the risk of severe early neurocognitive toxicity have brought the role of radiotherapy into question. With meningioma, the use of normal tissue-sparing techniques such as radiosurgery has allowed for the successful treatment of patients who are eminently curable and with a life expectancy that is generally no different than that of the general population. Particular attention in this review is paid to current approaches, contemporary trials, and modern therapeutic dilemmas.
Perhaps the most challenging therapeutic dilemma in the use of radiotherapy for brain tumors is the balance between tumor control and the tolerance of the normal tissues. Radiation oncologists have long referred to this issue as the therapeutic ratio. Radiation therapy has been found to improve several clinical endpoints in brain tumors, such as overall survival in glioblastoma, progression-free survival in low-grade glioma, and local control in atypical meningioma. However, radiation therapy has been implicated in significant delayed toxicities such as radiation-induced neurocognitive dysfunction and radionecrosis. A significant amount of the ongoing research on the use of therapeutic radiation in the treatment of brain tumors is being done to improve the therapeutic ratio between therapeutic gain and treatment toxicity. This review examines the current standards of care in common adult brain tumors and addresses current controversies and ongoing trials.
Current treatment paradigm in glioblastoma
Radiation therapy has long been the standard adjuvant approach for glioblastoma, and it remains the primary treatment modality in unresectable glioblastoma. A dose-response analysis performed by Walker in the late 1970s on patients treated in the previous Brain Tumor Cooperative Group Trials had established the standard radiation dose to be 60 Gy. This seminal analysis evaluated patients treated at 4 separate dose levels between 45 and 60 Gy and found a statistically significant improvement in survival in the 60-Gy cohort. Since then, several randomized comparisons have been published to support that 60 Gy is biologically superior to lower doses.
Given the advantage of escalating radiation dose to 60 Gy, numerous subsequent trials have attempted to further escalate the dose. But the limiting factor has been the ability of the brain to tolerate higher doses of radiotherapy. Because of the dose-volume relationship that predicts for toxicity of radiation therapy, one strategy to further increase dose was to decrease the irradiated volume. The classic treatment portals for glioblastoma encompassed the whole brain. Randomized trials have since shown that partial brain radiotherapy was no different from the previous whole brain portals in outcome. Patterns of failure data have shown that local failure within 2 cm of the enhancing tumor was the predominant mode of recurrence, whether or not the entire brain was treated. Strategies to decrease the treatment volume and increase the local radiotherapy dose were, however, unable to improve outcome. Several dose-escalation studies using hyperfractionation in an attempt to improve the therapeutic ratio were similarly unable to improve outcome. Moreover, no randomized trial has ever shown an advantage of treating to a higher dose than 60 Gy. A single-institution series from the Massachusetts General Hospital evaluating the ability of proton beam radiotherapy reported the escalation of radiation doses to 90 cobalt Gy equivalent and these doses were able to prevent tumor recurrences within the volume that received 90 Gy. This series marked the first report of the pattern of failure of glioblastoma being changed secondary to higher doses of radiotherapy. This series, however, was marked by high rates of symptomatic radionecrosis, highlighting the tenuous nature of the therapeutic ratio for glioblastoma. The series did suggest that if the 90-Gy dose could be successfully reached, the failure pattern could potentially be altered.
The advent of temozolomide chemotherapy has changed the treatment paradigm for glioblastoma over the past decade. In a trial published by the European Organisation for Research and Treatment of Cancer (EORTC), 2-year overall survival was improved from 10.4% to 26.5%. The update of the trial, which was presented at the American Society for Therapeutic Radiology and Oncology national meeting in 2007, demonstrated that the survival advantage persisted even after 4 years. Since the EORTC trial, the standard postsurgical treatment for newly diagnosed glioblastoma has become concurrent temozolomide (75 mg/m 2 ) therapy with radiation therapy (60 Gy), followed by 6 monthly cycles of temozolomide (150–200 mg/m 2 ) therapy.
Current management controversies
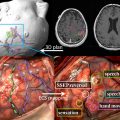
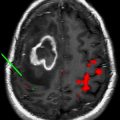
The optimal radiation treatment volume represents one of the major controversies in the radiotherapeutic management of glioblastoma. There are 2 currently accepted paradigms, each with a slightly different philosophy. In the United States, the Radiation Therapy Oncology Group (RTOG) treatment volumes are the accepted standard. The first 46-Gy dose is delivered to the peritumoral edema with a 2-cm margin. The final 14-Gy booster dose is delivered to the enhancing tumor with a 2.5-cm margin. The US treatment standard is based on biopsy data from several single-institution series that demonstrate tumor involvement extending into the peritumoral edema. Because the volume of the peritumoral edema is often significantly larger than the volume of the enhancing tumor, the treatment volume is reduced at 46 Gy to spare toxicity. In Europe, however, a 2- to 3-cm margin to the enhancing tumor alone is treated to 60 Gy, without any field reduction. The logic behind this treatment philosophy is that 80% of treatment failures occur within 2 to 3 cm of the enhancing volume. Furthermore, glioblastoma as a radioresistant tumor is unlikely to be sterilized by a 46-Gy preboost dose when compared with more sensitive tumors in other organ sites such as the breast and gastrointestinal tract. The next EORTC trial will be addressing this issue specifically and stratifying patients depending on the radiotherapy volumes in an attempt to determine if there may be any difference in the failure patterns. Fig. 1 depicts the controversy in treatment-volume delineation for glioblastoma.
A second issue to be addressed in the future of glioblastoma management with radiotherapy is the optimal imaging modalities for radiation-target delineation. At present, the RTOG accepted standard is the use of T2 or fluid-attenuated inversion recovery magnetic resonance imaging (MRI) sequences to identify peritumoral edema. T1 sequence with contrast images is used to identify the enhancing portion of the tumor. Several series have suggested that the use of metabolic imaging such as positron emission tomography, MR diffusion and MR perfusion, and MR spectroscopy may result in different treatment volumes than the traditional MRI sequences. Further advances in imaging may ultimately change the paradigm of tumor delineation.
Although the concept of dose escalation has been investigated in the past, several advances in the delivery of radiotherapy and in the development of effective drug therapy have suggested that this question be reconsidered. Previous dose-escalation trials were done before the advent of temozolomide. In solid tumors outside of the central nervous system (CNS), the use of concurrent chemotherapy has been shown to have a synergistic effect on local control. It may be that the addition of concurrent chemotherapy to a dose-escalation approach may allow a decrease in the radiation dose necessary to prevent a failure in the high-dose region. Furthermore, bevacizumab, which is presently being investigated as an up-front antiangiogenesis agent, has been implicated in decreasing the rate of radionecrosis caused by radiotherapy. The addition of such an agent in the up-front setting, as is being evaluated in the next RTOG trial, may ultimately allow for higher doses of radiotherapy without the previously seen dose-limiting toxicities. The incorporation of newer treatment modalities such as intensity-modulated radiation therapy (IMRT) and proton beam radiotherapy may allow for better sparing of normal tissues or better-tolerated dose escalation. The tolerance of the brain to radiotherapy is a dose-volume relationship, and with techniques such as proton beam radiotherapy and IMRT, the integral dose may potentially be decreased. The success of dose escalation may ultimately be related to the ability to incorporate the newer imaging techniques to correctly identify tumors on imaging, so that the highest-risk regions for failure can be selectively treated to higher doses.
Current management controversies
The optimal radiation treatment volume represents one of the major controversies in the radiotherapeutic management of glioblastoma. There are 2 currently accepted paradigms, each with a slightly different philosophy. In the United States, the Radiation Therapy Oncology Group (RTOG) treatment volumes are the accepted standard. The first 46-Gy dose is delivered to the peritumoral edema with a 2-cm margin. The final 14-Gy booster dose is delivered to the enhancing tumor with a 2.5-cm margin. The US treatment standard is based on biopsy data from several single-institution series that demonstrate tumor involvement extending into the peritumoral edema. Because the volume of the peritumoral edema is often significantly larger than the volume of the enhancing tumor, the treatment volume is reduced at 46 Gy to spare toxicity. In Europe, however, a 2- to 3-cm margin to the enhancing tumor alone is treated to 60 Gy, without any field reduction. The logic behind this treatment philosophy is that 80% of treatment failures occur within 2 to 3 cm of the enhancing volume. Furthermore, glioblastoma as a radioresistant tumor is unlikely to be sterilized by a 46-Gy preboost dose when compared with more sensitive tumors in other organ sites such as the breast and gastrointestinal tract. The next EORTC trial will be addressing this issue specifically and stratifying patients depending on the radiotherapy volumes in an attempt to determine if there may be any difference in the failure patterns. Fig. 1 depicts the controversy in treatment-volume delineation for glioblastoma.
A second issue to be addressed in the future of glioblastoma management with radiotherapy is the optimal imaging modalities for radiation-target delineation. At present, the RTOG accepted standard is the use of T2 or fluid-attenuated inversion recovery magnetic resonance imaging (MRI) sequences to identify peritumoral edema. T1 sequence with contrast images is used to identify the enhancing portion of the tumor. Several series have suggested that the use of metabolic imaging such as positron emission tomography, MR diffusion and MR perfusion, and MR spectroscopy may result in different treatment volumes than the traditional MRI sequences. Further advances in imaging may ultimately change the paradigm of tumor delineation.
Although the concept of dose escalation has been investigated in the past, several advances in the delivery of radiotherapy and in the development of effective drug therapy have suggested that this question be reconsidered. Previous dose-escalation trials were done before the advent of temozolomide. In solid tumors outside of the central nervous system (CNS), the use of concurrent chemotherapy has been shown to have a synergistic effect on local control. It may be that the addition of concurrent chemotherapy to a dose-escalation approach may allow a decrease in the radiation dose necessary to prevent a failure in the high-dose region. Furthermore, bevacizumab, which is presently being investigated as an up-front antiangiogenesis agent, has been implicated in decreasing the rate of radionecrosis caused by radiotherapy. The addition of such an agent in the up-front setting, as is being evaluated in the next RTOG trial, may ultimately allow for higher doses of radiotherapy without the previously seen dose-limiting toxicities. The incorporation of newer treatment modalities such as intensity-modulated radiation therapy (IMRT) and proton beam radiotherapy may allow for better sparing of normal tissues or better-tolerated dose escalation. The tolerance of the brain to radiotherapy is a dose-volume relationship, and with techniques such as proton beam radiotherapy and IMRT, the integral dose may potentially be decreased. The success of dose escalation may ultimately be related to the ability to incorporate the newer imaging techniques to correctly identify tumors on imaging, so that the highest-risk regions for failure can be selectively treated to higher doses.
Current trials
There are 2 major phase III trials underway, regarding the use of adjuvant radiotherapy in the treatment of glioblastoma. The RTOG 0825 trial is investigating the use of bevacizumab in the up-front setting with concurrent temozolomide and radiation therapy. Bevacizumab is a monoclonal antibody against vascular endothelial growth factor. The basis of the RTOG 0825 trial is the high response rates seen in a phase II trial performed by Duke Medical Center in the salvage setting when bevacizumab was used in combination with irinotecan. The objective response rate was 57%, with 46% of the patients without progression at 6 months. A subsequent randomized phase II trial comparing bevacizumab monotherapy with a combination of bevacizumab and irinotecan revealed that bevacizumab had activity as a single agent.
The second major phase III trial is the EORTC CENTRIC (Cilengitide, Temozolomide, and Radiation Therapy in Treating Patients With Newly Diagnosed Glioblastoma and Methylated Gene Promoter Status) trial that randomizes patients to the standard temozolomide-radiotherapy combination with and without cilengitide. Cilengitide is a selective α v -integrin inhibitor that has antiangiogenic properties. Two phase II trials have thus far been reported in an abstract form using concurrent cilengitide therapy in the up-front setting with temozolomide therapy and radiotherapy. In the New Approaches to Brain Tumor Therapy (NABTT) 0306 trial presented at American Society of Clinical Oncology (ASCO) 2009, 89 of 112 patients who were enrolled in the trial were alive at 12 months from diagnosis. Table 1 provides a summary of contemporary trials for glioblastoma.
| Trial | Adjuvant Therapy | Status |
|---|---|---|
| EORTC 26981 | 60 Gy 60 Gy/TMZ a + 5d TMZ b | Published in 2005 in the New England Journal of Medicine ; improved 2-y overall survival with TMZ. |
| RTOG 0525 | 60 Gy/TMZ a + 5d TMZ b 60 Gy/TMZ a + 21d TMZ c | Closed to accrual; awaiting preliminary analysis |
| RTOG 0825 | 60 Gy/TMZ a + 5d TMZ b 60 Gy/TMZ/bev d + 5d TMZ b | Open to accrual 2008 |
| EORTC CENTRIC | 60 Gy/TMZ a 60 Gy/TMZ/CLG e + 5d TMZ b | Open to accrual 2009 |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree