Fig. 1
Magnetic resonance imaging (MRI) demonstrating extracapsular spread on axial image (green arrow) (a) and seminal vesicle involvement on coronal image (orange arrows) (b)
Some patients may not be optimal candidates for EBRT and may be better suited for other modalities. These include patients with bilateral hip replacements in whom accurate CT-based treatment planning is technically challenging due to prosthesis artifact. In such cases, MRI-based simulation or the use of CT-MRI fusion techniques can be used to define the target more accurately with less artifact interference. Alternatively, ultrasound-guided brachytherapy may be selected where the prostate can be visualized more precisely. It is critical when planning EBRT to be able to deliver a therapeutic dose to the prostate while minimizing excess dose to the bowel and bladder. Patients with excessive small bowel adjacent to the prostate target volume or those who have received prior courses of pelvic radiotherapy for non-prostate cancer malignancies such as rectal cancers or testicular seminoma may not be able to receive tumoricidal dose levels of external beam radiotherapy safely and may be better managed with alternative modalities, i.e., brachytherapy.
3 Dose Escalation
EBRT for prostate cancer has evolved dramatically since the mid-1980s. Traditionally, bony landmarks were used to delineate field boundaries designed to encompass the pelvic lymph nodes, prostate and seminal vesicles (also known as a 4-field “box” technique). Using these fields, large volumes of normal tissue were irradiated. This limited the radiation dose that was safely deliverable to the prostate to 64 to 70 Gy and doses above these levels were associated with a significant morbidity risk, particularly with respect to the rectum and bowel (Smit et al. 1990). Newer techniques which use three-dimensional treatment planning and delivery such as 3D CRT and IMRT have refined EBRT markedly allowing more precise treatment delivery leading to more effective treatment with improved tumor control and morbidity outcomes.
Five randomized trials have been reported to date demonstrating a clear advantage to dose-escalated EBRT in terms of biochemical control and are discussed further in another section. Although there remains some controversy over which patients benefit most from dose escalation, it appears that intermediate and higher risk groups may derive a larger benefit (Kim et al. 2012; Pollack et al. 2002).
Despite improvements achieved by dose escalation with 3D CRT, increasing radiation dose came at a cost. Approximately 15–35 % of patients receiving doses ≥70 Gy developed grade ≥2 rectal toxicity (Dearnaley et al. 2007; Kuban et al. 2008; Zietman et al. 2010). In the M.D. Anderson randomized trial comparing 70 and 78 Gy, grade ≥2 gastrointestinal toxicity rates were observed in 13 and 23 % of patients, respectively (Pollack et al. 2002). Peeters et al. reported an increased rate of rectal bleeding requiring laser/transfusion (p = 0.07) in patients treated to 78 Gy compared with 68 Gy (Peeters et al. 2006). Urinary morbidity has been less consistently altered by dose escalation with some studies showing similar outcomes (Michalski et al. 2010) and some showing worse toxicity with dose escalation (Dearnaley et al. 2007).
In order to further establish the benefits of dose escalation, the Memorial Sloan-Kettering Cancer Center initiated a phase I/II protocol in 1988, whereby the prescription dose was increased from 64.8 to 86.4 Gy in successive increments of 5.4 Gy (Leibel et al. 1994; Zelefsky et al. 1998). In 1996, IMRT was introduced as a therapeutic tool to overcome the increased rectal toxicity seen with dose escalation using 3D-CRT at doses ≥75.6 Gy. The first report demonstrating the potential benefits of IMRT over 3D conformal approaches was published by Zelefsky et al. in 2000 (Zelefsky et al. 2000). In that study, 61 patients received 81 Gy with 3D-CRT and 171 patients received the same dose with IMRT. Twenty randomly selected patients were planned with both IMRT and 3D-CRT. Toxicity and dosimetric analyses comparing the two techniques demonstrated that IMRT resulted in better coverage of the CTV by the prescription dose than 3D-CRT (p < 0.01) and reduced the volumes of rectal and bladder walls receiving 75 Gy (p < 0.01). Clinically, this translated into a reduced rate of late grade 2 and 3 rectal bleeding in the IMRT group (2 vs. 10 %, p < 0.001). In 2002, Zelefsky et al. reported the safety of delivering doses of 81 Gy to the prostate using IMRT in a larger cohort of 772 patients (Zelefsky et al. 2002). In that series, the 3-year actuarial likelihood of developing grade ≥2 late genitourinary and gastrointestinal toxicity was 15 and 4 %, respectively. Although at the time follow-up was limited, IMRT resulted in at least equivalent biochemical control rates as non-IMRT approaches, quelling concerns that IMRT may result in underdosing of the target due to constricted dose distributions. In a recent update of these data with a median follow-up of 7 years, the 8-year likelihood of developing grade ≥2 late genitourinary and gastrointestinal toxicity was 15 and <2 %, confirming the long-term safety of this treatment (Cahlon et al. 2008). In addition, biochemical control rates remained excellent with 8-year actuarial PSA relapse-free survival rates for patients in favorable, intermediate, and unfavorable risk groups of 89, 78, and 67 %, respectively (p = 0.0004).
Others have found similar benefits of dose-escalated IMRT. Vora et al. reported biochemical and toxicity outcomes of dose-escalated IMRT (75.6 Gy) compared with conventional doses (68.4 Gy) in a series of 272 patients and found a 14 % improvement in 5-year biochemical control in the high dose group (Vora et al. 2007). Higher dose IMRT was well tolerated as there were no significant differences in toxicity between the two groups. In a series of 133 patients treated with 74–76 Gy using IMRT reported by De Meerleer et al., toxicity was also noted to be low with 17 and 19 % of patients experiencing late grade 2 GI and GU toxicity, respectively (De Meerleer et al. 2007). Additionally, biochemical outcome was excellent with 3-year biochemical control rates of 100, 94, and 74 % for low-, intermediate-, and high-risk patients, respectively.
The most updated analysis of the MSKCC dose escalation experience has recently been reported including over 1,000 patients treated with IMRT to a dose of 86.4 Gy (Spratt et al. 2013). With a median follow-up of 5.5 years, the 7-year biochemical relapse-free survival rates were 98.8, 85.6, and 67.9 % for low-, intermediate-, and high risk patients, respectively. Late toxicity was minimal with <1 and 2.2 % of patients experiencing late grade 2 or higher GI and GU toxicity, respectively. The rate of acute grade 2 of higher GI and GU toxicity was 4.4 and 21.1 %, respectively.
4 Techniques of IMRT Treatment Planning and Delivery
The development of three-dimensional treatment planning techniques has significantly improved the accuracy of EBRT. Dedicated treatment planning CT and MRI scanners in radiation oncology simulation suites allow the capture of complete volumetric anatomical information which can be directly imported into the treatment planning software. Radiation therapy treatment plans are generated by a careful definition of the target and normal tissues from which customized beams can be individually shaped allowing better shielding of organs at risk. The dose distributions can then be analyzed either graphically in a dose–volume histogram (DVH) or on axial, sagittal, and/or coronal CT images. By using volumetric parameters to evaluate dose, a more careful and complete analysis of target and normal tissue dose distribution is achievable.
4.1 IMRT Treatment Planning
Three-dimensional (3D) conformal radiotherapy uses a forward treatment planning process, whereby the dosimetrist selects beam specifications (e.g., direction, weight, shapes, and modifiers) and calculations are made based on these settings. A trial and error-based approach is used to select the best dose distribution from these specifications. With IMRT, a mathematical approach called inverse planning is used. In this process, the target dose and coverage are chosen, and then normal tissue dose and volume constraints are loaded into the computer program. An optimization algorithm modifies the intensity profile of each radiation beam and the specific shape of each treatment field or aperture in an iterative fashion until the desired dose distribution is achieved. Each beam is nonuniform such that the intensity of the beam varies across the treatment field. By modulating the intensity of each beam, a steep dose gradient is created between the PTV, rectum, and bladder, typically allowing at least 85–90 % of the PTV to receive the prescription dose while maintaining rectal and bladder doses within established tolerances. Doses to organs overlapping the PTV such as the bladder and rectum are often constrained to receive <100 % of the prescription. Given the steep falloff of the dose gradient, accurate targeting becomes increasingly important when implementing an IMRT plan. Treatment planning studies have shown improved conformality of dose distributions around the PTV compared with 3D conformal treatment planning (De Meerleer et al. 2000; Ling et al. 1996).
During the past 10–15 years, many techniques for IMRT treatment have been implemented (e.g., ‘step and shoot’, sliding window, helical fan beam, volumetric modulated arc therapy (VMAT)), although they all share a goal of creating a concave dose distribution by using multiple coplanar fields arranged at each or nearly equal spacing about the patient. At MSKCC, a 5–7 field beam arrangement is used to encompass the PTV to dose levels of 81 Gy or higher (Fig. 2). Other techniques include arc-based treatments or specialized tomotherapy units which provide similar but slightly different dose distributions (Fig. 3).
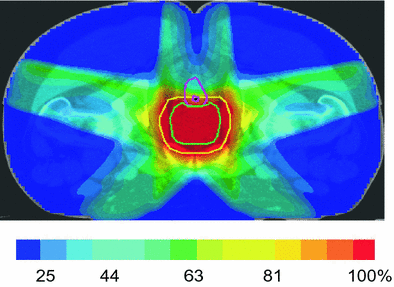
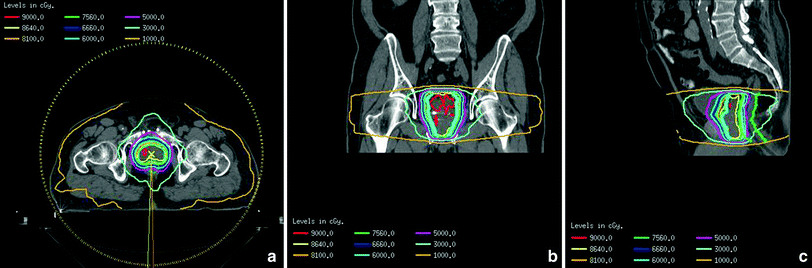

Fig. 2
Dose distribution (% prescription dose) for a 5-field IMRT plan

Fig. 3
Isodose distribution of VMAT (volumetric modulated arc therapy) plan a axial view, b coronal view, c sagittal view
4.2 IMRT Treatment Delivery
In order to optimize the use of 3D conformal radiotherapy and/or IMRT, improved methods of recognition and correction of geometric uncertainties such as setup error and organ motion are necessary to integrate into treatment delivery. Organ motion during a course of radiation therapy introduces the potential for day-to-day variations in the position of the prostate (interfraction motion) as well as during a 15–20 min treatment session (intrafraction motion). Image-guided approaches have provided a means to account for target motion during radiation therapy and are discussed further in other chapters. Briefly, several methods of image-guided external beam radiotherapy are available and in the current clinical use. One of the simplest techniques includes the use of implanted gold fiducial markers which can be visualized on two-dimensional X-ray images obtained immediately prior to daily treatment from which positional corrections can be made. Other methods include an ultrasound-based system to detect and correct prostate, bladder, and rectum positioning using B-mode acquisition and targeting (BAT; Nomos Corp., Sewickley, PA). The most recent and the most complex approach includes the use of linear accelerators equipped with CT capability which can be co-registered with treatment planning CT scans.
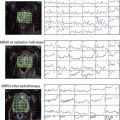
At the Memorial Sloan-Kettering Cancer Center, we routinely use intraprostatic fiducial marker placements to guide the IMRT treatment. The placement of three radio-opaque (typically gold) markers into the prostate via a transrectal ultrasound-guided approach provides a simple way of tracking motion of the prostate during radiotherapy. The fiducial markers are placed prior to simulation and digitally reconstructed radiographs (DRR images) can be created on which the markers can be clearly seen (Fig. 4). Daily imaging using an electronic portal imaging device allows accurate pretreatment detection, verification, and correction of the prostate position. Several studies have shown this technique to have less user variability than ultrasound-based methods, which require specialized training (Langen et al. 2003). Marker placement is generally well tolerated and marker migration is relatively rare (Trichter and Ennis 2003). We recently reported the toxicity profiles and biochemical outcomes from a cohort of 186 patients undergoing high-dose image-guided radiotherapy (IGRT) for localized prostate cancer which were retrospectively compared with a similar cohort of 190 patients treated with non-image-guided therapy (nIGRT) (Zelefsky et al. 2012). For the IGRT cohort, the rate of grade 2 or higher urinary toxicity was significantly less (10.4 vs. 20.0 %; p = 0.02) with no significant differences seen in the rectal toxicity or biochemical outcomes.
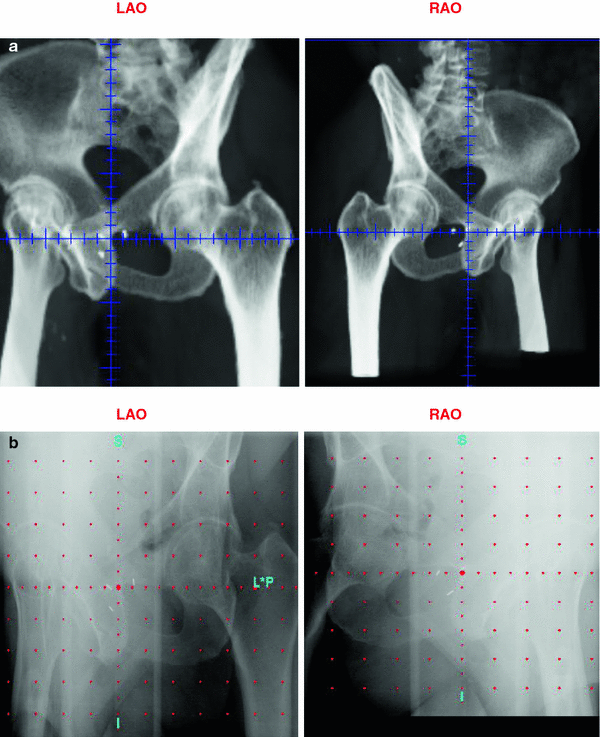

Fig. 4
Fiducial markers placed in prostate visible on left anterior oblique and right anterior oblique digitally reconstructed radiograph (a) and portal image (b)
A new system has been developed which allows for monitoring of prostate motion during treatment delivery (Calypso Medical Technologies Inc, Seattle, WA). This system uses transponders, also placed transrectally, which emit an electromagnetic signal when excited. Detection of the signal by an alternating current magnetic array localizes the transponders in real-time. The radiation beam may be turned off should the target stray out of the radiation field or beyond a preset motion constraint. Unpredictable intrafraction motion shifts which would otherwise be unaccounted for are detectable and able to be corrected with this system. By increasing the accuracy of treatment delivery in this way, smaller PTV margins may be feasible and possibly further improve toxicity profiles, although this is yet to be clinically established.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree