, Foster D. Lasley2, Indra J. Das2, Marc S. Mendonca2 and Joseph R. Dynlacht2
(1)
Department of Radiation Oncology, CHRISTUS St. Patrick Regional Cancer Center, Lake Charles, LA, USA
(2)
Department of Radiation Oncology, Indiana University School of Medicine, Indianapolis, IN, USA
Definitions
Alpha (α) particle: A particle emitted by the nucleus through α decay, containing 2 protons and 2 neutrons.
Identical to a helium nucleus.
Beta (β) particle: A particle emitted by the nucleus through β decay, either negatively or positively charged.
β − particle: basically an electron but from the nucleus. (“negatron”, normal matter).
β + particle: A positron. (antimatter, positively charged version of electron)
Gamma (γ) ray: A photon emitted by the nucleus. (different from an x-ray which is from an electron interaction but may have the same energy range).
Internal conversion electron: Emitted when a nucleus transfers energy to an orbital electron instead of emitting a gamma ray.
Characteristic X-ray: A photon emitted by an electron transitioning from one shell to another.
Other than the source of production, a 30-keV X-ray is identical to a 30-keV γ − ray.
Formalism: Decay Schemes
Radioactive decay may be described by a reaction scheme, such as:
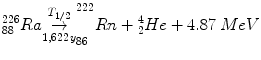
(2.1)
These schemes allow us to track mass, charge, and energy.
Mass, charge and energy are always conserved. In order to gain charge a nucleus must emit a negative charge, etc.
Decay energy is shared between the decay products, depending on the mode of decay.
In α − decay, almost all of the energy goes into the α − particle.
In γ − decay, all of the energy goes into the photon (or an internal conversion electron, in internal conversion).
In β − decay, energy is shared between a beta particle and a neutrino or an antineutrino.
Alpha (α) Decay
The nucleus is bloated, proton heavy and wants to lose weight!
The “pairing rule” states that particles are more stable in pairs.
Therefore, nuclei emit alpha particles (2 neutrons and 2 protons) instead of single protons or neutrons.
Occurs in very heavy (Z > 52) nuclei, such as:
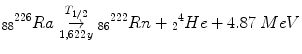
(2.2)
Decay energy is split between the daughter nucleus and the alpha particle, but almost all of it goes to the alpha particle.
Typical alpha energies range from 2 to 8 MeV.
Alpha particles are monoenergetic.
Beta (β) Decay
Occurs in nuclei that need to gain or lose protons, such as:
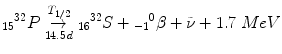
(2.3)
Beta decay is always isobaric (see Chapt. 1), meaning there is no change in atomic mass number.
Unlike alpha decay there is only minimal mass change.
There are three different beta decay modes:
Beta-minus and beta-plus actually produce beta radiation.
Electron capture is a “beta” process but produces gamma radiation.
Beta particles are polyenergetic because the energy is shared between the beta particle and the neutrino/antineutrino.
Neutrinos and antineutrinos do not really interact with regular matter, so they do not contribute to radiation dose.
The average energy of a beta particle is approximately 1/3 of the maximum energy.
Beta-Minus (β−) or Negatron Emission
The nucleus is proton-poor and wants more protons!
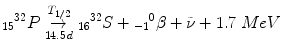
(2.4)
Thanks to weak nuclear force interactions, one of the neutrons is able to turn into a proton.
Atomic number goes up by 1; mass stays the same.
In doing so, the nucleus spits out an electron and an antineutrino.
The electron is also known as the β − particle or negatron.
When you create matter (β − ) you also create antimatter (antineutrino).
Beta-Plus (β+) or Positron Emission
The nucleus is proton-rich and wants more neutrons!
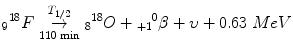
(2.5)
One of the protons turns into a neutron, basically the exact opposite of beta-minus decay.
Atomic number goes down by 1; mass stays the same.
It spits out an anti-electron (positron) and a neutrino.
Positrons are antimatter with mass! When the positron meets a regular electron, it annihilates!
This releases the electron and positron’s mass as energy.
The rest mass of an electron is 0.511 MeV; so two identical 0.511 MeV photons are emitted in opposite directions. This is useful for imaging (PET scans).
Since the positron carries 1.02 MeV of annihilation energy, it costs 1.02 MeV to make the positron.
Therefore, the kinetic energy is 1.02 MeV less than if the particle had decayed by electron capture.
Electron Capture (EC)
The nucleus is proton-rich and wants more neutrons!
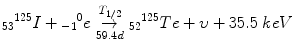
(2.6)
When nucleus is proton rich but does not have an excess of 1.02 MeV, the nucleus eats one of its electrons.
During electron capture it emits a neutrino, but most of the decay energy remains in the daughter nucleus.
This energy is immediately emitted as a gamma ray or as internal conversion electrons.
(see section “Gamma Emission”)

Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree