Fig. 3.1
Schematic set up of liver RF treatment with assumed current pathways
Elevated temperature is able to impair the cells via several ways. The primary manner of cell death during thermal ablation for tumor is acute coagulative necrosis. When temperature is up to 41°C, it causes the blood vessel dilation and the increase of blood perfusion. Meantime, it can stimulate the production of heat-shock protein for protecting the cell from heat injury and repairing the damage tissue. Nevertheless, this temperature-stimulated protective mechanism is associated with heat intensity and heat exposure time. When temperature is up to 46°C and heat exposure is up to 10 min, irreversible necrosis of significant cell mass will produce. Cells recovery from heat injury may exhibit an increased tolerance to elevated temperatures. Temperatures at 46–52°C will attenuate the time to induce cell death and begin to trigger microvascular thrombosis, ischemia, and hypoxia. This reactive cascade cuts off the supply of cells’ nutrient and leads to delayed cell necrosis. Temperatures in excess of 60°C will rapidly cause protein denaturation and melt the plasma membrane, which is essential for cells survival [7, 8].
3.1.2 The Development of RFA
Three periods were defined since RFA was applied to manage of HCC in clinical practice according to the development of radiofrequency electrodes. The first phase was in the early 1990s when a single and solid-center needle electrode was used in RFA and only for the lesions with a size of 1.6 cm in diameter. Therefore, it was utilized infrequently in the clinic by the restriction of ablative size. The second phase was in the middle of 1990s when the electrode was greatly modified. Then, multiple electrodes and cooled needle electrode were invented, as represented by LeVeen electrodes (Radiotherapeutics) and internally cooled electrodes (Radionics), respectively. Both of them are capable of enlarging the ablative size to 3.5–5.0 cm in diameter with dramatic improvements of therapeutic efficacy. With the comprehensive application of RFA in the treatment of HCC, it is gradually becoming the typical measure of local ablative therapy and attracts more and more attention in the field of liver surgery. The third-generation electrodes were developed by integrating the advantages of needle electrode and saline-enhanced electrode. For example, Celon Power (Olympus) has integrated two to three kinds of the second-generation electrodes so as to further increase the ablative region to 5.0–7.0 cm in diameter. Furthermore, we can apply multi-electrode ablation system to accurately position the electrodes based on the tumor’s shape, then precisely realize the “conforming ablation,” all of which will additionally improve the therapeutic efficacy of RFA [9, 10].
3.1.3 Commonly Used Commercial Available RFA Systems
There are a variety of RFA systems commercially available today, which can be divided into three categories based on their principles: temperature-controlled, impedance-controlled, and temperature-impedance-controlled operating mechanisms. There are also many kinds of electrode needles including extendable needle, single needle, multiple-needle, and needles with intrinsic/extrinsic cycle perfusion. Each RFA system usually has several matched electrode needle types with their own advantages and disadvantages. We currently introduce the most popular commercial available RFA systems as follows: Radionics™ (Radionics Medical, Boston, MA), RITA® (Rita Medical, Mountain View, CA), Radiotherapeutics™ (Radio-Therapeutics, Sunnyvale, CA), Berchtold® (Berchtold, Tuttlingen, Germany), and CelonPOWER system (Celon AG Medical Instruments, OLYMPUS, Japan). All of them were frequently reported in publications [11–14].
3.1.4 Radionics™ RF System
The Cool-tip™ System (Radionics Burlington, MA, Fig. 3.2a) works at impedance control mode and 200-W power source pulses energy delivery with frequency of 480 kHz to generate the larger ablation volumes. The exclusive feedback algorithm continuously monitors the tissue impedance and automatically adjusts the maximum energy delivery. Thermocouple at electrode tip scrutinizes the tissue temperature. Once the therapeutic cycle completed, the electrode can be repositioned to measure the temperature at ablation zone.
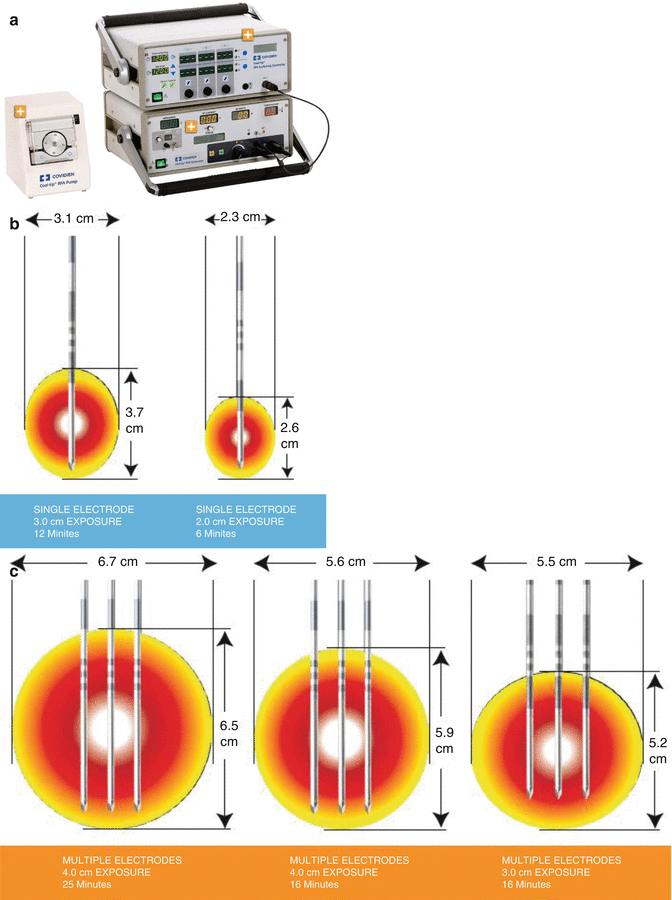

Fig. 3.2
(a) The Cool-tip™ System. (b) The Cool-tip™ needles. (c) Generating a larger ablative lesion with three electrodes simultaneously
Their needles (Fig. 3.2b) are all straight, internally cooled electrode design. Each needle is sized 1.6 mm in diameter with a 2.0–4.0 cm active distal part (Fig. 3.2b). This RF system requires four neutral pads, two on the pelvis and one on the hind thighs, respectively. The ablative zone sized 2.5–4.0 cm in diameter can be created with single needle by one operation (Fig. 3.2b). Also, we can use three electrodes simultaneously to generate the larger ablative area sized up to 6 cm in diameter (Fig. 3.2c).
3.2 RITA® RF System
The Model 1500™ (RITA® Medical Systems, Mountain View, CA) is a RF system with a 150-W generator and operates at 460-kHz frequency (Fig. 3.3a). The expandable electrode (StarBurst™ XL; Rita Medical Systems) consists of an insulated outer needle sized at 2.2 mm in diameter that houses nine deployable curved tines. When the electrodes are fully extended, the maximum diameter can reach to 5 cm, which mimics the configuration of a Christmas tree (Fig. 3.3b). In order to control the generator, five of the nine prongs containing thermal sensors are responsible to measure the tip temperature. The power is controlled in accordance with an average temperature of these probes. Electrodes were progressively extended deeper into the liver parenchyma, with temperature monitoring, to represent the standard algorithm widely used with this system. The standard method allows the maximum energy to be applied until the mean temperature reaches the threshold. Tines were deployed firstly to 2 cm at a pre-selected target temperature of 80°C, then advanced to 3 cm at a targeted temperature of 105°C, and finally extended to 4–5 cm at a targeted temperature of 110°C. The targeted temperature should be maintained for 7 min prior to measuring the post-ablation temperatures with five thermocouples. For this system, two grounding pads were required to place on the thighs.
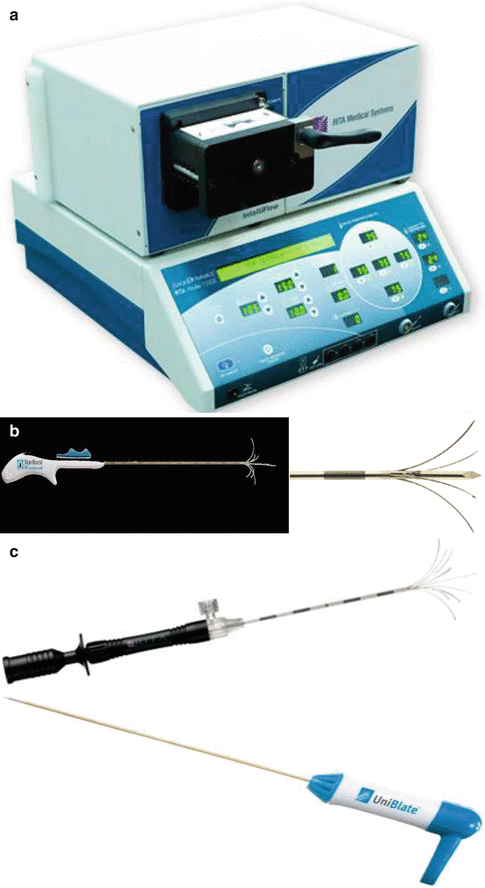

Fig. 3.3
(a) The Model 1500™ (RITA® Medical Systems, Mountain View, CA). (b) The StarBurst™ XL needle (RITA® Medical Systems, Mountain View, CA). (c) Other type of needles for RITA® RF Systems
There are also several other type needles available for RITA® RF system (Fig. 3.3a), but their ablation zone is smaller than that induced by StarBurst™ XL.
3.2.1 Radiotherapeutics™ RF System
The RF3000™ system (Radiotherapeutics, Sunnyvale, CA) utilizes a maximum power of 200-W generator with an operating frequency of 480 kHz (Fig. 3.4a). This system is used with a 12-prong LeVeen™ multitined array electrode (Fig. 3.4b). The 2.5-mm-diameter cannula of the electrode contains 12 retracted curved distal tines, which when fully expanded form an umbrella shape, 4.0–5.0 cm in maximum diameter, perpendicular to the axis of the probe. After the deployment of tines, the power output is initially triggered at 80 W and then escalated by 10 W every 30 s until it reaches 130 W. Without the rise of impedance over 200 Ω after 5 min at 130 W, power output is increased by 10 W every 30 s up to 190 W and is continuously maintained until either a 15-min application time elapsed or an uncontrolled impedance rise is observed. After 30 s, a second circle is started and RF energy is applied until the appearance of next uncontrolled impedance rise. In case uncontrolled impedance rise occurs at the 130-W initial power in the first 5 min, power is re-applied thereby after 30 s with 50 % reduction. Subsequent power output is escalated by 10 W every 30 s up to 190 W and is continuously maintained until either the entire 15-min application time elapsed or of next impedance rise happened. There is no need for changing the probe position; these two applications are of the capacity to create an ablation area with this system. Four grounding pads are required for each procedure, as recommended by the manufacturer. Two pads are attached on the pelvis, and one is placed on the hind thighs, respectively.
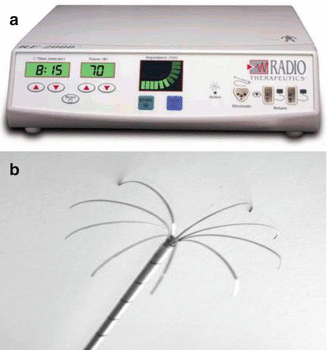

Fig. 3.4




(a) The RF3000™ system (Radiotherapeutics, Sunnyvale, CA). (b) LeVeen™ multitined array electrode
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree