Postoperative paraspinal fluid collections can present a management dilemma to both radiologists and surgeons. Although many of these collections present as incidental findings and are unrelated to the presenting signs and symptoms that led to the imaging study, certain collections in the context of the appropriate clinical scenario may require additional evaluation and even emergent intervention. This article reviews those collections that are most frequently encountered and suggests management strategies that may assist in the evaluation and management of the patient.
Key points
- •
Paraspinal fluid collections are often seen after spine surgery. This increased frequency is likely due to surgical manipulation, but may also reflect other factors such as the extent of the spine surgery, patient comorbidities, and patient coagulation status.
- •
Postoperative imaging modalities include myelography, ultrasound, radionuclide scanning, computed tomographic scan, and magnetic resonance imaging. Magnetic resonance imaging remains the best study to visualize and define the spectrum of paraspinal fluid collections.
- •
Management of paraspinal fluid collections is challenging. Communication between the radiologist and surgeon is extremely helpful in managing these complex situations.
Introduction
The high prevalence of back and/or neck pain in the United States population is estimated at 20%. A large number of these patients initially or eventually undergo spinal surgery. Approximately 1.2 million spine surgeries, including 300,000 spinal fusion surgeries, are performed each year in the United States. It is always important to try to understand why an operative intervention was performed. The indications for spine surgery include deformity correction for congenital scoliosis, relief of neural compression from space-occupying lesions such as disc herniation, osteophyte formation, epidural mass or hematoma, relief of spinal canal stenosis due to degenerative or infectious spine disease, or correction of spinal instability. This information may be helpful in understanding why a potential complication occurs. For example, an analysis of non-neurologic complications following spinal surgery for adolescent idiopathic scoliosis in 702 patients showed that hematoma, seroma, and dehiscence occurred in 0.71% of the surgeries. These latter complications were associated with increased blood loss and prolonged operative and anesthesia times. In addition, knowledge of the specific type of spinal surgery, whether it is deconstructive such as a laminectomy for spinal stenosis or reconstructive such as a fusion for instability, can be helpful in the search for abnormal paraspinal fluid collections. The radiologist should be familiar with the types of surgical approaches, tools, implants, and techniques associated with spine surgeries as this will assist in identifying focal abnormalities. Approaches may consist of anterior, posterior, or combined anterior-posterior and may use instrumentation such as screws, spinal wires, artificial ligaments, vertebral cages, artificial discs, and osteoinductive agents. Different spine fusion techniques are associated with different complications and complication rates.
Although most spine surgeries are successful, complications may be observed. Postoperative spine paraspinal fluid collections are not uncommon. They may or may not be symptomatic, but their presence, either as an incidental finding or as a possible etiologic agent in the postoperative patient’s clinical presentation, requires careful evaluation and management. Given their frequent occurrence, they have received limited attention in the medical literature, yet often pose a diagnostic dilemma for both surgeon and radiologist alike. The purpose of this article is to describe the various types of paraspinal postoperative fluid collections with respect to their imaging findings, to characterize them, and to suggest possible management strategies.
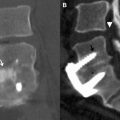
Different types of fluid collections may be encountered within or about the spinal axis following spine surgery. Fortunately, the overwhelming majority of these collections are self-limited and resolve with conservative management. There are those paraspinal fluid collections, however, that are found on postoperative spine imaging evaluation in symptomatic patients that may require further diagnostic evaluation, such as percutaneous aspiration, or additional surgical intervention such as open decompression and/or medical management with prolonged antibiotic therapy ( Fig. 1 ). The fluid collections may be classified by location, intra-spinal or within the epidural compartment, or paraspinal, that is anterior, dorsal, or lateral to the spinal axis. These collections may also be classified by type and include hematoma, seroma, pseudomeningocele, and abscess ( Table 1 ). It is very important for the radiologist to be aware of when the surgical procedure was performed, not only to better understand the imaging manifestations of the collection, but also to determine whether prior preoperative and postoperative imaging examinations are available for comparison. Hematomas may be seen acutely after spine surgery; abscesses may be seen subacutely, and seromas and pseudomeningoceles may be seen as subacute or chronic processes.
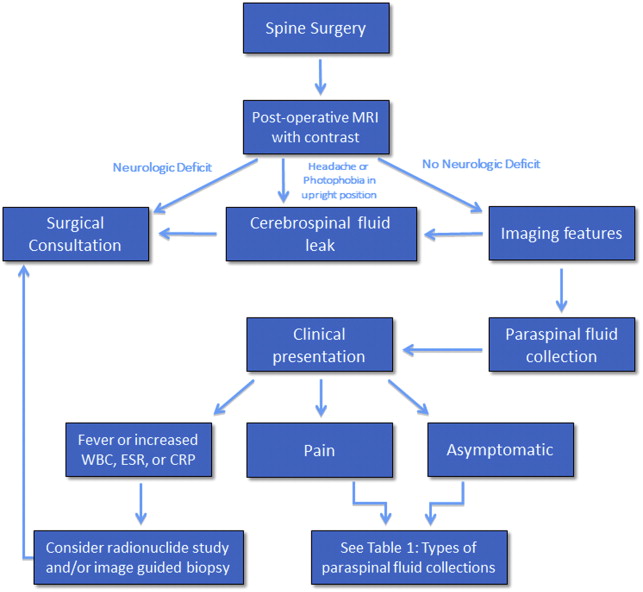
| Hematoma | Intraspinal, paraspinal |
| Seroma | Paraspinal |
| Pseudomeningocoele | Paraspinal |
| Abscess | Intraspinal, paraspinal |
Hematoma
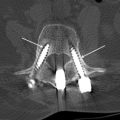
A hematoma is an extravascular collection of blood of variable size and extent that can, postsurgically, occur within or outside of the spinal canal. These focal hemorrhages may result from surgical manipulation and damage to blood vessels in the operative field. They are uncommon and occur in less than 1% of spine surgery patients, in 5.4% of patients undergoing posterior lumbar fusion, and in 5.6% of anterior cervical discectomy and fusion cases. Patients with coagulation deficits, either intrinsic or related to anticoagulation medication, or with multilevel spine surgery, may also be at increased risk for developing postsurgical hematomas. Hematomas occur acutely and, depending on the size, location, and extent of the hemorrhage, can present in the acute or early subacute postsurgical period. The clinical presentation may occur within hours to days after spine surgery. Patients may present with increasing, severe sharp pain with or without a radiating component. Patients may also present with focal neurologic deficits especially when the hematoma is located in the epidural space. It is therefore essential to perform an imaging study urgently in a postoperative spine patient with this type of clinical presentation ( Table 2 ). Anterior paraspinal hematomas may compress the upper aerodigestive tract, resulting in dysphagia and/or respiratory distress.
| Definition | A contained intraspinal and/or paraspinal hemorrhagic collection that can be found anywhere along the course of the operative tract |
| Imaging findings | Acute: hyperdense to isodense on CT; hypointense to isointense on T1 and heterogeneously hypointense to hyperintense on T2 MR imaging; mild peripheral enhancement on MR imaging Subacute: hypodense on CT; increased signal intensity on T1 and T2; mild peripheral enhancement; hematocrit level may be present on either study Chronic: hypodense on CT; fluid signal on T1 and T2 MR imaging with residual foci of low high signal on T1 and low signal on T2 |
| Management | Asymptomatic patient: observation and imaging surveillance Symptomatic patient: Epidural hematoma: surgical consultation for probable evacuation Paraspinal: image-guided percutaneous or surgical drainage |
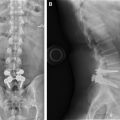
The imaging study of choice to evaluate a patient with a suspected hematoma in the epidural space is magnetic resonance (MR) imaging. The MR imaging appearance of an acute epidural hematoma is that of a somewhat heterogeneous hypointense to isointense fluid collection on T1-weighted sequences and a heterogeneous hypointense to hyperintense fluid collection on T2-weighted sequences. Gradient echo sequences may show foci of hypointensity. Delayed hematomas may show increased signal intensity on both T1-weighted and T2-weighted images. If present within the epidural space, the collection may extend for several vertebral body segments and may be associated with spinal cord impingement and spinal cord edema ( Fig. 2 ). Hematomas that are located outside of the spinal canal, within or adjacent to the paraspinal muscles, may show signal changes on T1-weighted and T2-weighted sequences that parallel the evolution of blood breakdown products. Indeed, over time, a hematocrit level may be seen, such that with more chronic hematomas, it may be impossible to distinguish them from seromas ( Fig. 3 ). Thin peripheral contrast enhancement may be observed. On computed tomographic scan (CT), hematomas, depending on their age and location, will manifest as increased, intermediate, or low attenuation. They can be difficult to detect in the acute phase, as the attenuation may be comparable with that of other adjacent soft tissue structures.
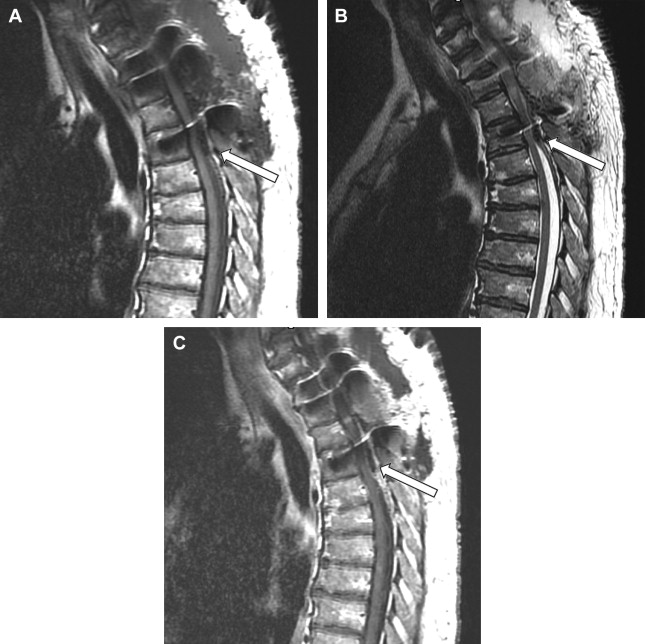
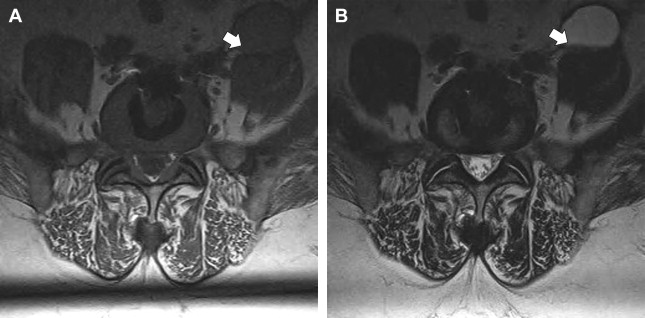
Epidural hematomas often require immediate surgical consideration for evacuation. A large epidural hematoma in a symptomatic patient will likely require surgical decompression with laminectomy and removal of the hematoma. Smaller and more chronic-appearing epidural or paraspinal hematomas are often managed conservatively and may require imaging surveillance to assess for any interval change in size and appearance. In asymptomatic patients with chronic-appearing small (less than one vertebral segment) hematomas or seromas that are gradually involuting, it may be prudent to avoid percutaneous aspiration as this procedure may predispose to superinfection of a sterile collection. Percutaneous aspiration of paraspinal hematomas may be considered in patients in whom symptoms may be referable to the collection or in whom the collection is already suspected to be infected. In this situation, the procedure should be performed with strict aseptic technique with subsequent careful monitoring and follow-up of the patient. Hematomas in the subdural space following spine surgery are rare and more often associated with procedures such as lumbar puncture or spinal anesthesia.
Seroma
A seroma is a fluid collection that contains lymphatic fluid and may or may not be surrounded by a fibrous capsule. The presence of a seroma reflects disruption of local lymphatic vessels. Given the close proximity of lymphatics to arterial, capillary, and venous structures, it is not uncommon to have some disruption of these structures during surgery and to have a relatively lesser amount of hemorrhage accumulate within the seroma as well. This accumulation of hemorrhage may result in a hematocrit level within the seroma and may account for the overlap in the literature with respect to classification; seromas and hematomas are often considered similar entities. Seromas can be located anywhere along the path of surgical intervention and may be seen subcutaneously or adjacent to the spinal canal or paraspinal soft tissue structures. Postoperative spine seromas are to be distinguished from Morel-Lavallee seromas; the latter are seen after blunt closed soft tissue trauma or after abdominal reconstructive or plastic surgery. The Morel-Lavallee seromas are located subcutaneously and often respond to treatment with compression bandages ( Table 3 ).
| Definition | A fluid collection that contains lymphatic fluid and may or may not be surrounded by a fibrous capsule |
| Imaging findings | CT: hypodense paraspinal fluid collection; MR imaging: fluid signal on T1-, T2-, and diffusion-weighted images. Mild peripheral enhancement on MR imaging. A small amount of debris or hemorrhage may layer dependently within the collection |
| Management | Small seromas tend to be asymptomatic and can be observed Larger seromas can be associated with symptoms Treatment options: compression bandages, image-guided percutaneous or surgical drainage, especially when possibility of infection is clinically suspected |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree