and Ashutosh Dash2
(1)
Nuclear Security and Isotope Division, Oak Ridge National Laboratory, OAK RIDGE, USA
(2)
Isotope Production and Applications Division, Bhabha Atomic Research Centre, Mumbai, India
14.1 Introduction
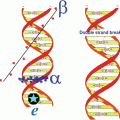
One of the most widely used applications of therapeutic radioisotopes is for the treatment of inflammatory (rheumatoid) joint disease (Fig. 14.1) caused from destruction of diarthrodial or synovial tissues in individuals (Otero and Goldring 2007).
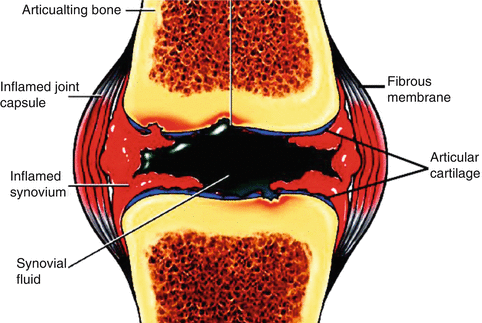

Fig. 14.1
Schematic of the a synovial joint
Intra-articular administration of β− emitting radionuclides in the form of colloidal formulation or radiolabeled particulate (preferably of 1–10 μm size range) into the articular cavity, referred as “radiation synovectomy (RSV)” or “radiosynoviorthesis,” has emerged as an effective option of treatment and a viable alternative to surgical synovectomy. RSV is defined as the restoration (orthesis) of the synovia by the local application of radiolabeled particulates or radionuclide-loaded colloid particles. The biological mechanism of RSV essentially involves the absorption of radioactive particulates by superficial cells of the synovium followed by phagocytosis by the macrophages of the inflamed synovium. Beta radiation (Fig. 14.2) results in coagulation necrosis, fibrosis, and sclerosis of the proliferating synovial tissue and leads to destruction of diseased pannus and inflamed synovium (Deutsch et al. 1993). This prevents the secretion of fluid and accumulation of inflammation, causing cellular compounds and the joint surfaces to become fibrotic, offering rapid and sustain pain relief. The effective cytotoxic radiation dose required to be delivered to the joints is determined principally by the size of the joint along with other factors such as synovial thickness, synovial structures (smooth, villous fine/rough edematous), condition of the joint fluid (watery or gelatinous), and inflammatory activity of the synovium (Kitonyi and Kitonyi 2009; Karavida and Notopoulos 2010; Özcan 2014; Dunn et al. 2002) (Fig. 14.3).
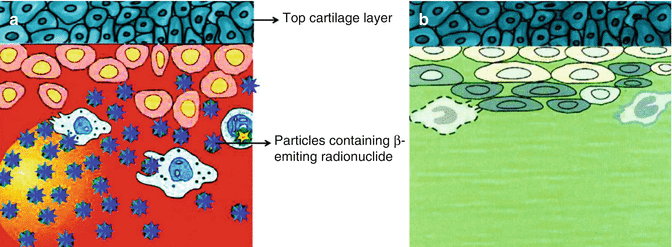
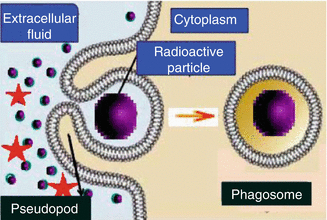

Fig. 14.2
(a) Particles containing β−-emitting radionuclide (blue stars) phagocytized by inflamed hypertrophic synovial lining with proliferating synoviocytes without affecting top cartilage layer. (b) Successive cell damage and sclerosis of synovial membrane

Fig. 14.3
Phagocytosis where cell sequester radioactive particulates by extension of pseudopodia and vesicle formation
14.1.1 Advantages of Radiosynovectomy
Minimally invasive intervention.
Radiosynovectomy is generally performed as an outpatient procedure and involves overnight hospital stay.
No surgical/anesthetic risk.
Provides an attractive treatment option for inoperable patients.
Intensity and duration of rehabilitation is minimal.
Effective radiation dose for the treatment is low.
Multiple joints may be treated simultaneously or at short intervals.
Multiple radioactive dose administration to achieve maximum response.
Relatively free of side effect.
14.1.2 Selection of Radionuclides
While a myriad of factors contribute to the utility of RSV, selection of an appropriate β−-emitting radionuclide is critical since the synovial thickness of different joints in the human body (e.g., finger, wrist, knee, etc.) varies substantially. Also, joints at different stages of the disease show different degrees of swelling and may also call for a beta emitter of different penetration. The β− radiation energy should be sufficient to penetrate and ablate proliferating layer of the inflamed synovium with minimum radiation-induced damaged to the underlying articular cartilage or adjacent bone underneath (Chakraborty et al. 2015) (Fig. 14.4). The radionuclide dose should be small enough to be phagocytosed, and it should be given in amounts that are limited enough so that leakage does not occur from the joint (Schneider et al. 2005). The radionuclide should have a half-life suffice for adequate, but not excessively prolonged, to deliver cytotoxic radiation dose to the synovium and should be substantially less than the retention time of the radiolabeled particle in the joint to be treated. The penetration depth of the β− radiation energy should correspond to the thickness of the synovium in the joint to be treated, as inadequate penetration will give an inferior therapeutic effect and excessive penetration depths may constitute a safety hazard. As the thickness of the diseased membrane varies in different joints, treatment of diseased synovium in joints of disparate size requires radionuclide of different β− particle energies with high-energy beta emitters such as 90Y and 188Re for the large joints such as the knees and the 169Er low beta emitter for treatment of the finger joints. A medium beta energy emitter such as 186Re is useful for irradiation of medium-sized synovial joints such as the elbow. The other important issues are leakage of the radioisotope form the joint, which must be minimized by the characteristics of the preparation, and the gradual particle adsorption by the synovium. The half-life should be long enough to allow good distribution within the synovium and adequate exposure, while being short enough to avoid excessive irradiation and significant leakage from the joint. There is no evidence that the use of radioisotopes is a therapeutic option for the treatment of osteoarthritis (Moskowitz 2007).
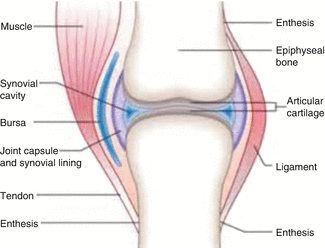

Fig. 14.4
Diagram illustrating the anatomical relationship of the synovial membrane to other structures in the knee joint and needle placement for introduction of radioisotope preparations for synovectomy
Table 14.1 summarizes the principal radioisotopes and preparations which have been evaluated or which are in current use as approved radiopharmaceutical agents for the treatment of rheumatoid arthritis. These agents are approved for clinical use in many countries, but interestingly, radiation synovectomy is not practiced in the USA and these agents have not yet been approved by the regulatory authorities, presumably because of the potential ramifications of joint leakage, although extensive studies as discussed later in this chapter have demonstrated the safety and efficacy of this method. The first reports of this last treatment were published as early as 1924 (Ishido 1924) and subsequent later (Fellinger and Schmid 1952) and paved the way for the contemporary broad clinical application of radionuclide synovectomy.
Table 14.1
Examples of radioisotope preparations developed and evaluated for treatment of inflammatory (rheumatoid) joint disease
Radioisotope | Half-life Major E βmax (Int%) | Therapeutic range (X90) in the synovium | Major joints | Radiopharmaceutical (particle size μm) | Key references |
|---|---|---|---|---|---|
Radioisotope preparations which have been evaluated for treatment of synovitis | |||||
Gold-198 | 2.7 days 0.960 (98 %) | 0.39 mm | Large (knee) | High gamma energy | |
Dysprosium-165 | 2.3 h 1.285 (83 %) | 0.57 mm | All | Ferric hydroxide macroaggregates (FHMA) (0.8–12) Only low gammas | Hnatowich et al. (1978) McLaren et al. (1990) Sledge et al. (1986) |
Holmium-166 | 26.8 h 1.773 (48 %) 1.854 (51.0 %) | Medium, large | Chitosan FHMA Polylactic acid (PLA) microspheres (1.2–12) | Cho et al. (2012) Argüelles et al. (2003) Makela et al. (2004) Mupmer et al. (1992) | |
Lutetium-177 | 6.71 days 0.497 (78.7) | 1.3 | Small | Hydroxyapatite (HA) | Herrick et al. (1994) |
Phosphorus-32 | 14.29 days 1.701 (100 %) | 2.1 | All | Chromic phosphate | Sohaib et al. (2011) |
Rhenium-188 | 16.9 h 2.12 (71.4 %) | 2.1 | Large (knees) | Sulfide/microspheres | Liepe et al. (2011) Wang et al. (2001) |
Samarium-153 | 46.7 h 0.632 (3.1 %) 0.702 (44.1 %) | 1.6 | Large | HA macroaggregates | |
Approved radiopharmaceuticals for routine clinical treatment of synovitis | |||||
Erbium-169 | 9.5 days 0.350 (55 %) | 0.18 | Small | Citrate | |
Rhenium-186 | 3.7 days 1.076 (71 %) | 1.2 | Medium | Heptasulfide colloid | Klett et al. (2007) |
Yttrium-90 | 2.7 days 2.26 (99.9 %) | 2.22 | Large (knee) | Citrate/silicate High beta energy (1.5–3.5) No gamma photons | |
So the challenges in developing a useful agent for the treatment of synovitis include the selection of the appropriate radionuclide(s). In addition to radionuclidic properties, another key issue is availability and radionuclides used for this application decay by beta emission and thus are generally reactor-produced (Table 14.1). One example which can be either reactor or accelerator produced is 186Re (see Chaps. 6 and 7). Key radionuclidic properties include emission of a beta particle with the requisite energy which matches the joint dimensions and required soft tissue penetration requirements (Table 14.2). For radiation safety considerations, these radioisotopes should decay without emission of significant abundance of accompanying high-energy gamma photons. However, the emission of gamma photons which can be imaged is important to substantiate homogeneous distribution within the joint space and to evaluate the possibility of any leakage of activity.
Table 14.2
European Commission recommended activity doses of approved radiopharmaceutical agents for joint synovectomy
Treated joint | Administered activity, MBq | Recommended volume (ml) | ||
|---|---|---|---|---|
169Er | 186Re | 90Y | ||
Large joints – yttrium-90 | ||||
Knee | 185–222 | 3–5 | ||
Medium joints – rhenium-186 | ||||
Ankle (tibiotalar) | 74–185 | 1–1.5 | ||
Elbow | 74–111 | 1.2 | ||
Foot (subtalar) | 37–74 | 1.15 | ||
Shoulder (glenohumeral) | 74–185 | 4 | ||
Wrist | 37–74 | 1–1.5 | ||
Small joints – erbium-169 | ||||
Fingers (metacarpophalangeal) | 20–40 | 1 | ||
Foot (metatarsophalangeal) | 30–40 | 1 | ||
Hands/feet (proximal interphalangeal) | 10–20 | 0.5 | ||
An additional important characteristic is the availability of a particulate preparation which is stable for a sufficient time period after administration prior to synovial resorption. As summarized in Table 14.1, there are many therapeutic radioisotopes which have been evaluated for radionuclide synovectomy. Selection of particulate for use in RSV is primarily based on the ability for facile and reproducible preparation and demonstration of effective synovial targeting/uptake. The particulates should also be sufficiently robust enough to withstand the radiolabeling reaction conditions and should exhibit chemical characteristics which must form a stable complex with the radionuclide and should exhibit good stability to circumvent loss of the radioisotope from radiolysis or during application. The particle chemical characteristics should permit simple and efficient radiolabeling, and they should be nontoxic, nonallergenic, biocompatible, and preferably biodegradable. The binding between the radionuclide and the particle should be irreversible throughout the course of the radiotherapy. Another prerequisite is that the particle must be sufficiently small to be phagocytized; however it should not be too small that would allow joint leakage before phagocytic trapping. The appropriate medium major diameter size range for this application is considered to be from 2 to 10 μm. Following intra-articulation, the radiolabeled particles should be distributed homogeneously in the joint without initiating an inflammatory response.
There are a variety of materials and particulate preparations used for synovectomy, which include preparations based on citrate, sulfide colloids, hydroxyapatite particles, and chitosan (Karavida and Notopoulos 2010; Teyssler et al. 2013; Kasteren et al. 1993; Davis and Chinol 1989; Song et al. 2001; Brecelj et al. 2007). Ideally, the particle size distribution should be in the 2–10 μm range. Depending on the particles, it is possible to incorporate the activity, label throughout the entire volume, or label only in certain structures, such as the surface and the outer or inner wall. The binding of radioactivity to particles can be done by covalent bonds, by chelation, by adsorption processes, or by indirect means. In the light of the perceived need to preclude leakage of the radioactive isotope from the microsphere, the prospect of enclosing the nonradioactive precursor of the radioisotope in the microsphere matrix and subsequent activation in a nuclear reactor shortly before use seemed attractive.
There are of course regulatory guidelines which must be followed for the use of such agents for treatment of synovitis and Table 14.2 summarizes guidelines used in the European community. It is interesting to note that although the first development and the first demonstration of clinical efficacy was reported in the USA (Sledge et al. 1986), radiation synovectomy is not practiced in the USA evidently due to a regulatory concern that potential joint leakage of the radioisotope would expose non-target tissues to unacceptable radiation dose.
14.2 Dosimetry and Dose Rate
Although a detailed discussion of dosimetry is beyond the goal of this presentation, these studies are important and can provide important guidance on the use of radionuclide for synovectomy. In one recent particularly important study, the use of erbium-169 (169Er), phosphorus-32 (32P), rhenium-188 and samarium-153 (188Re and 153Sm), and yttrium-90 (90Y) were analyzed, since these are radionuclides of current major interest for radiosynovectomy (Torres et al. 2009). The therapeutic range in synovial tissue values and the adsorbed dose values per activity of injected radionuclide (Gy/h*MBq) profiles to the synovial membrane and juxtaposed articular cartilage were evaluated in detail. These useful data provide estimates of the dose expected to be delivered to the synovial membrane and cartilage for joints of different dimensions and for different stages of the arthritic process, which present with different degrees of swelling. In this context, it is recommended in European theater that MRI imaging be initially conducted to determine the tissue volume and the appropriate radionuclide for synovectomy treatment which is dependent on the synovial thickness for treatment and the proximity of the bone and cartilage non-target tissues. The RS procedure demands efforts of a well-coordinated multidisciplinary team of medical specialists, including musculoskeletal physicians, radiologists, nuclear physicians, hematologists, physical therapists, and appropriately trained nurses. In the modality, the role of radiologist represents the key in the assessment of the suitability of the target joint.
14.3 Key Therapeutic Radioisotopes Used for Synovectomy
Many of the radioisotopes described in this section are also used for a variety of other therapeutic applications using unsealed sources described in this book and the production and processing details are included in Chaps. 5, 6, and 7.
14.3.1 Dysprosium-165
In more contemporary times, 165Dy is the first radiopharmaceutical agent which stimulated interest in the more widespread introduction of this technology for the treatment of refractory synovitis as an alternative to surgical intervention (Hnatowich et al. 1978; Sledge et al. 1986; McLaren et al. 1990). It is interesting to note an unusual commentary that the use of 165 Dy as the first therapeutic radioisotope for intra-articulation for this application was developed by Prof. Sledge in Boston. In spite of this pioneering work and effectiveness of this treatment, the US Food and Drug Administration (FDA) has not approved such use on the basis of the dosimetric implications of “potential” capsule leakage. Thus, although this use of therapeutic radioisotopes is practiced essentially worldwide except in the USA for effective treatment of such conditions—also in hemophilic patients—it is not routinely practiced in the USA.
14.3.2 Erbium-169
Because of the more abbreviated anatomical dimensions, the use of low-energy beta emitters is indicated for the treatment of synovitis of the small joints in order to minimize any possible radiation damage to the underlying bone or other tissues. The use of reactor-produced 169Er as the citrate is thus the current preferred option for synovectomy of small joints (Kahan et al. 2004; Menkes et al. 1977). A review of the use of 169Er for the treatment of inflamed small joints has been recently published (Karavida and Notopoulos 2010).
14.3.3 Gold-198
The first papers on the use of 198Au in the treatment of arthritis appeared in 1963 in which chronic effusions of the knee was treated with 370 MBq of gold-198 colloids (Ansell et al. 1963). Few studies on the use of 198Au have been reported in the literature (Cayla 1967; Topp and Cross 1970; Van Soesbergen et al. 1988; Visuthikosol and Kumpolpunth 1981). Although used in the early development of the use of therapeutic radioisotopes for synovectomy, 198Au is no longer used for this indication, primarily because of the emission of high-energy gamma photons (411 keV, 95 %).
14.3.4 Holmium-166
One early strategy for the preparation of 166Ho-labeled agents for this application involved preparation of biodegradable neutron-activated poly-l-lactic acid (PLA) microspheres (2–13 μm) (Mupmer et al. 1992). The microspheres were prepared by combination of PLA and nonradioactive 165Ho-acetyacetonate (166Ho-AcAc) in ChCl3 which was then added to 3 % polyvinyl alcohol. After stirring, the precipitated microspheres (2–13 nm) were centrifuged and the microspheres obtained by 3.0 μm filtration, treated with HCl to remove any excess AcAc, and the solution then repeatedly filtered through 3.0 μm filters. The 165Ho-AcAc microsphere target material was then irradiated at a thermal neutron flux of about 8.88 × 1012 n/cm2·s. Evaluation after intra-articular injection into the knee joint space of rabbits demonstrated about 98 % retention with no leakage after 120 h. The initial studies in a small patient group with 166Ho-FHMA involved an average 1.11 GBq administration into the knee joints of 22 patients in which 17 patients (71 %) showed complete or partial response (Ofluoglu et al. 2002). Although some joint leakage was observed by detection of low levels of activity in local inguinal lymph nodes in 6 treatments, the authors concluded that this as a safe and effective method for treatment of synovitis.
Hydroxyapatite [HP; Ca10(PO4)6(OH)2] is an important natural component of teeth and bones and forms strong complexes by binding with many cations. HP particles labeled with 166Ho has also been widely evaluated for synovitis therapy and are prepared by combining 166Ho-citrate with HA by mixing and agitating at room temperature (Unni et al. 2002). Hydroxyapatite (HA) is a naturally occurring mineral form of calcium apatite with the formula Ca5(PO4)3(OH) but is usually written Ca10(PO4)6(OH)2 to denote that the crystal unit cell comprises two entities. Hydroxyapatite is the hydroxyl end member of the complex apatite group. The OH−ion can be replaced by fluoride, chloride, or carbonate, producing fluorapatite or chlorapatite. It crystallizes in the hexagonal crystal system. Pure hydroxyapatite powder is white. Naturally occurring apatites can, however, also have brown, yellow, or green colorations, comparable to the discolorations of dental fluorosis. Up to 50 % of bone by weight is a modified form of hydroxyapatite. Another approach has been the radiolabeling of the chitosan molecule, which is a linear polysaccharide and consists of randomly distributed deacetylated β-(1-4)-linked d-glucosamine and acetylated N-acetyl-d-glucosamine units. It is obtained by sodium hydroxide treatment of shells from shrimp, lobster, and clams and other crustaceans with sodium hydroxide treatment. Because of excellent electrostatic properties, chitosan has high metal cation-binding properties and has been used to bind a variety of therapeutic radioisotopes. The 166Ho-chitosan complex has also been evaluated for treatment of synovitis (Seong et al. 2005; Lee et al. 2003a, b).
14.3.5 Lutetium-177
More recently, the use of 177Lu for synovectomy has been explored. Advantages include the reactor production in very high activity levels and high specific activity (>70 Ci/g 176Lu at a thermal neutron flux of >1 × 1015 n/cm2/s) 177Lu, the emission of moderate energy beta particle (497 keV), and the emission of relatively low-energy gamma photons (208 keV, 11 %) which permit imaging to evaluate the homogeneity of joint distribution and possible leakage. Moreover, the relatively longer half-life of 177Lu provides logistic advantage for shipment to nuclear medicine centers far away from the reactors. The 177Lu preparations which have been evaluated for synovectomy include hydroxyapatite and liposomes. Increasing use of 177Lu for synovectomy has been impressive and widespread applications of 177Lu therapeutic agents have stimulated progress (Chakraborty et al. 2006, 2014a, b; Kamaleshwaran et al. 2014; Bard et al. 1985; Abbasi et al. 2011; Teyssler et al. 2013).
14.3.6 Phosphorus-32
32P has received attention due to its easy economic availability and reasonably satisfactory nuclear characteristics [T ½ = 14.3 days; E β (Max.) = 1.7 MeV; 3–5 mm of tissue penetration depth, respectively]. 32P chromic phosphate emerged as the current agent of choice in the USA and Canada. The utility of 32P is thus continually evolving and well entrenched in the arena of radiosynovectomy (Manco-Johnson et al. 2002; Onetti et al. 1982; Rivard et al. 1985, 1994; Siegel et al. 1994, 2001; Silva et al. 2001; Mortazavi et al. 2007). Samarium [32P] phosphate colloid has also recently been exploited as the agent for synovectomy (Prabhakar et al. 2007). The method of preparation involves the reaction of SmCl3 carrier with carrier added [32P]H3PO4 in the presence of gelatin. The pure colloid was recovered by dialysis.
14.3.7 Rhenium-186
Rhenium-186 is the radioisotope of choice for the treatment of inflammatory disease of the medium-sized joints such as the shoulder, elbow, wrist, hip, and ankle (Table 14.2). 186Re-sulfide is the radiopharmaceutical used for the treatment (Gedik et al. 2006; Tebib et al. 2004; Göbel et al. 1997; Kavakli et al. 2008). The 186Re-labeled sulfide colloid preparations are available from several commercial supplies as approved radiopharmaceutical preparations for the treatment of inflammatory joint disease (http://www.iba-molecular.com/products/re-186-mm-1). The recommended activity range for RSO with 186Re sulfide colloid is as follows: for the hip, 74–185 MBq; shoulder, 74–185 MBq; elbow, 74–111 MBq; wrist, 37–74 MBq; ankle, 74 MBq; and subtalar joint, 37–74 MBq (van der Zant et al. 2004). When several joints are being treated in a single session, the total activity administered should not exceed 370 MBq (EANM 2003; Farahati et al. 1999). 186Re-sulfide has been widely used in the clinical practice for 25 years in Europe, and its efficacy is well accepted in RA, while adverse effects are minor and infrequent. Reviews describing the clinical use of the 186Re colloid have been recently published (Klett et al. 2007; Koca et al. 2012).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree