Fig. 10.1
Port placement with a peri-umbilical skin incision (around 2.5 cm)
An 8.5-mm straight port for a camera, two curved 5-mm cannulas—crossing at the level of the abdominal wall—and a 5-mm straight laparoscopic trocar are introduced through this port. The robotic platform automatically switches the arm control to facilitate instrument control.
The procedure starts by retracting the gallbladder upward. The dissection of Calot’s triangle is performed using the monopolar hook (Fig. 10.2). Once the cystic artery and the cystic duct are dissected (critical view of safety) (Fig. 10.3), a Hemolock (Teleflex, Medical, Ireland) is placed on each side of the cystic artery (Fig. 10.4), and then on each side of the cystic duct (Fig. 10.5). The structures are divided using a robotic scissor. A retrograde dissection is performed (Fig. 10.6 and Video 10.1). The gallbladder is placed in a special endoscopic bag. The robot is dedocked and the specimen is retrieved (Fig. 10.7). The abdominal wall is carefully closed using an absorbable suture.
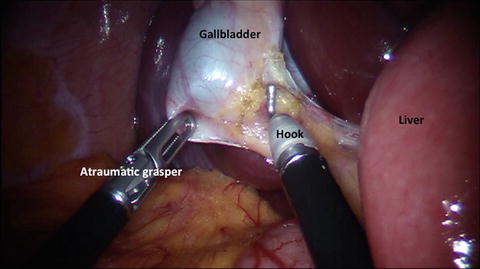
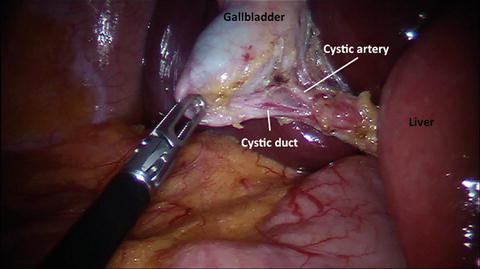
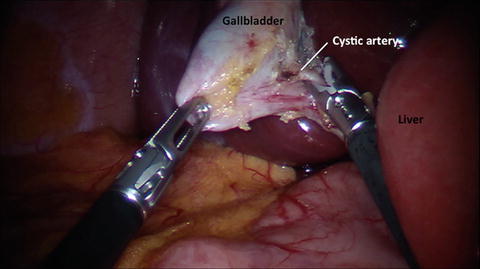
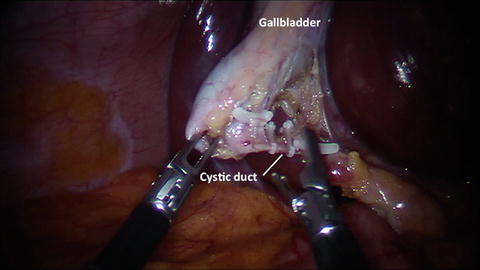
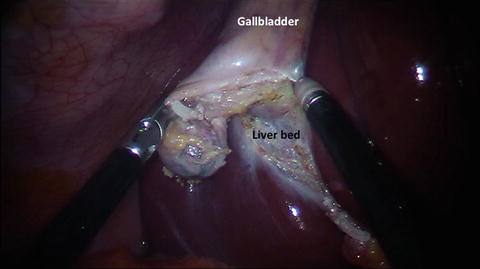
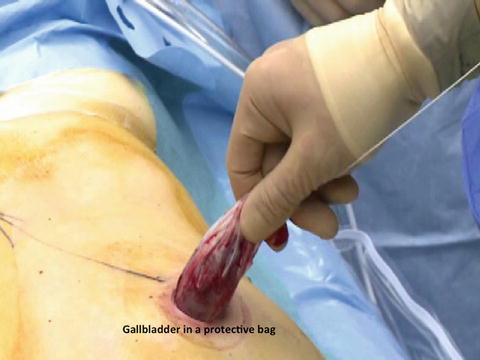

Fig. 10.2
Dissection of Calot’s triangle using a monopolar hook and an atraumatic grasper

Fig. 10.3
View of the cystic duct and the cystic artery

Fig. 10.4
Clipping of the cystic artery

Fig. 10.5
Clipping of the cystic duct

Fig. 10.6
Retrograde dissection of the gallbladder

Fig. 10.7
Retrieval of the specimen in a wound protector bag
The technique remains the same during a RSSC with fluorescent cholangiography, as reported previously [14]. To summarize, this fluorescence-capable da Vinci Si HD vision system is an endoscopic imaging system for high definition (HD) visible light and near-infrared fluorescence imaging. The system components include a surgical endoscope capable of visible light and near infrared imaging, a 3DHD stereoscopic camera head that couples to the endoscope and an endoscopic illuminator that provides visible light and near infrared illumination through the surgical endoscope via a flexible light-guide. The fluorescence-capable illuminator provides lighting for the surgical field. The system displays the live video image on the 3DHD stereo viewer and the touchscreen. If switched to fluorescence imaging, the Camera Control Unit processes and displays the resulting images as a fluorescent overlay on a black and white image. The surgeon can quickly switch between normal mode and fluorescence (NIR) by either making adjustments in the control menu at the console or initiation at the finger clutch [14].
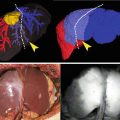
The ICG imaging agent is a sterile, water-soluble tricarbocyananine dye with a peak spectral absorption at 800–810 nm in blood plasma or blood. ICG is administered intravenously. ICG is nearly exclusively eliminated by the liver into the bile and does not undergo enterohepatic recirculation [34]. Physiologically, ICG appears unconjugated in the bile about 8 min after injection. ICG removal from the blood depends on liver blood flow, parenchymal cellular function, and biliary excretion. 2.5 mg of ICG is administered intravenously during patient preparation by anesthesia, approximately 30–45 min before the start of the case. A second dose of 2.5 mg ICG can be administered intravenously if fluorescence is not detected in the liver approximately 45 min after injection of the first dose or if additional questions regarding perfusion arise during surgery. The RSSC starts as described above. Once the usual retraction of the gallbladder cranially and laterally is achieved exposing the triangle of Calot, the camera view is switched to the fluorescence image (Fig. 10.8 and Video 10.2) and a first attempt to visualize the biliary anatomy can be performed. Then, the triangle of Calot is dissected in the usual fashion until the cystic duct and artery are skeletonized for at least 1 cm (Fig. 10.9 and Video 10.3). The camera view can be switched to the fluorescence image as desired to view the biliary anatomy during this surgery [14].
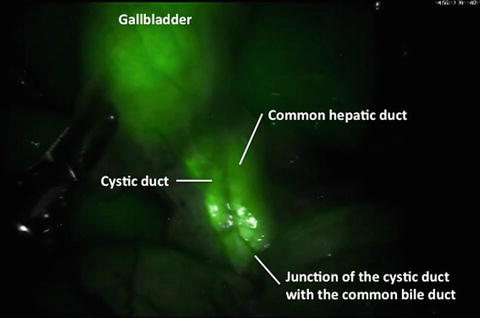
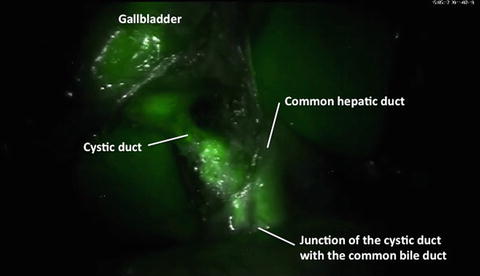

Fig. 10.8
Near-infrared fluorescent cholangiography before the dissection of the Calot’s triangle

Fig. 10.9
Near-infrared fluorescent cholangiography after the dissection of Calot’s triangle
Exploration of the Biliary Anatomy
Several groups have reported their experience with near-infrared fluorescent cholangiography using ICG. Although the feasibility and the safety seem well reported for a standard laparoscopic cholecystectomy [15, 17–19], the results for RSSC are scarcer. Indeed, only a couple of centers, including ours, have reported interesting and encouraging outcomes [14, 16].
As a potential advantage, the ICG administration is done before surgery and the biliary structures can be analyzed before any dissection, which is not the case for cholangiography requiring extensive dissection of the cystic duct. Moreover, the surgeons can obtain a road map of the biliary tree in real-time and at any time during dissection of Calot’s triangle [18]. Table 10.1 summarizes our current data regarding the use of ICG for biliary extrahepatic anatomy assessment.
Table 10.1
Visualization of biliary extrahepatic anatomy using near-infrared fluorescent cholangiography
Before dissection | After dissection | |
|---|---|---|
Cystic duct (%) | 91 | 100 |
Common hepatic duct (%) | 50 | 66.7 |
Common bile duct (%) | 33.3 | 83.3 |
Junction between cystic and common bile duct (%) | 25 | 58.3 |
Spinoglio et al. [16] found relatively similar results. Indeed, in a selected population of 45 patients, they reported different rates of visualization prior to Calot’s triangle dissection: 93 % for the cystic duct, 88 % for the common hepatic duct, 91 % for the common bile duct, and 88 % for the junction. After dissection, this rate rose up to 97 % for all the structures. As in our experience [14], at least one biliary structure was visualized in all patients [16].
The interest of a real-time biliary assessment remains to exclude a biliary anatomical variation as it was reported for standard laparoscopic cholecystectomy [35]. Regarding the robotic experience, Calatayud et al. [36] reported an interesting case of aberrant biliary canaliculus, identified by ICG. Obviously, this anatomical malformation could have been missed by a standard approach with potentially dramatic consequences.
Overall, using ICG during RSSC can help to assess the extrahepatic biliary anatomy and thus could reduce the risk of biliary injury by misinterpretation of the biliary anatomy. However, there are no available data to verify this hypothesis. On the other hand, it seems obvious that a clear interpretation of anatomy, especially in real-time and before any dissection, might prevent a biliary injury. Further studies are still required to define the exact role of real-time near-infrared fluorescent cholangiography during RSSC, even if the preliminary data are encouraging.
Other Potential Advantages
Considering the capability to analyze and recognize quickly the extrahepatic biliary anatomy, ICG fluorescent cholangiography might help to shorten the operative time. Indeed, a quick recognition of the cystic and common bile duct is a key step during a cholecystectomy.
Recently, we have shown that ICG fluorescent cholangiography was able to significantly shorten significantly the operative time in normal Body Mass Index (BMI) patients [37]. When considering patients with a BMI less than 25 undergoing RSSC with ICG in comparison to standard RSSC, the operative time was reduced by 24 min (p = 0.06). In addition, considering only patients undergoing RSSC with ICG, the operative time was shortened by 25 min in low BMI patients compared to patients with BMI >25 (p = 0.004) [37].
This interesting finding can be explained by the tissue penetration of near-infrared light. Indeed, ICG can be visualized only in a couple of millimeters (typically 5–10 mm) [18]. In obese patients, the Calot’s triangle is often covered by a thick layer of fat, which might limit a quick recognition of the main biliary structures and thus slow down the dissection phase. The same is true in the case of severe inflammation. Ishizawa et al. [18] reported that even in these challenging situations, fluorescent cholangiography can be helpful by frequently using fluorescent imaging during cystic dissection.
Inclusion and Exclusion Criteria
The current experience reported in the literature was undergone under IRB approval. While the near-infrared fluorescent cholangiography can help to assess the extrahepatic biliary anatomy, it is not validated to rule out a common bile duct stone and, thus strict selection criteria should be followed [14, 37].
Initially, the inclusion criteria included patients between 18 and 80 years old with symptoms consistent with gallbladder disease and gallstones confirmed by ultrasound, and with ASA score 1–3. Currently, age and ASA score are not strict but relative criteria. Of course, a preoperative proof of gallbladder disease remains mandatory.
On the other hand, initially the exclusion criteria were acute cholecystitis, biliary pancreatitis or suspicion of common bile duct stones, and/or perturbation of a liver test. Currently, acute disease does not represent a strict contraindication. In case of suspicion of common bile duct stone, a preoperative imaging ruling out this pathology should be performed. Of note, Ishizawa et al. [17] performed fluorescent cholangiography in 52 patients during standard laparoscopic cholecystectomy and showed that fluorescent images might identify gallstones in the cystic duct. However, they failed to diagnose common bile duct stones.
Moreover, previous upper abdominal open surgery was also considered as a relative contraindication. In addition, a history of adverse reaction to ICG or any contraindication to ICG label was also considered as reasons not to proceed with this approach. Even if the risk of allergic reaction remains low, caution should be undertaken before injection of ICG. In fact, ICG administration is reported to be safe and well tolerated, without any side effects in various robotic studies [14, 16, 37]. Previous reports [15, 18, 38] have confirmed the safety of this drug for several other indications. The risk of anaphylactic reactions is approximately 0.003 % at doses exceeding 0.5 mg/kg [39] and thus the risk remains quite small considering the small amount of ICG (2.5 mg) injected intravenously for cholangiography [18].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree