Fig. 1
Cause-specific survival in patients with and without prostate- specific antigen failure (adopted from Kwan et al. (Kwan et al. 2004))
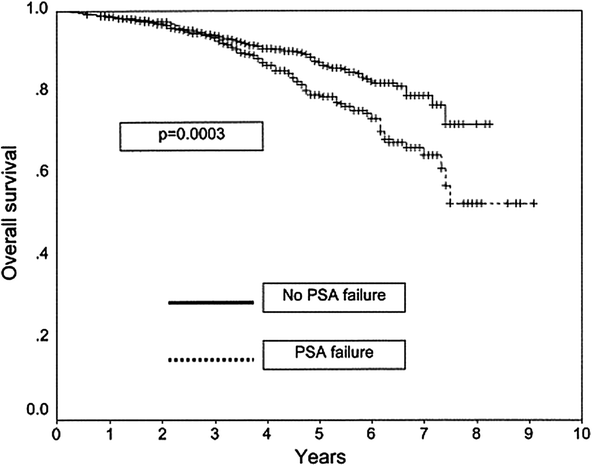
Fig. 2
Overall survival in patients with and without prostate- specific antigen failure (adopted from Kwan et al. (Kwan et al. 2004))
Previously, Soto and coworkers showed significantly better overall survival rates in biochemically failed patients with low-risk features and a pretreatment PSA velocity of ≤2 ng/mL/year compared to high-risk patients in biochemical recurrence (Soto et al. 2008). This emphasizes the idea that not all biochemical recurrence events are equivalent in terms of survival, and the need to determine whether biochemical recurrences are clinically significant and as such warrant further therapy. Thus, an increasing interest in analyzing the ability of additional prognostic factors such as the pretreatment initial prostate-specific antigen PSA, T stage, and Gleason score as well as PSA kinetics to predict for inferior prostate treatment outcomes, including survival has been generated.
4 PSA Recurrence: A Valuable Surrogate Marker in Clinical Trials?
The identification and rigorous validation of surrogate endpoints for overall or disease-specific survival are of great utility in clinical cancer research because the use of surrogate endpoints substantially decreases the size and duration of clinical trials allowing more rapid prospective testing of hypotheses and potentially accelerating the development of improvements in cancer treatment.
Established prognostic factors do not necessarily make valid surrogate end points. Surrogate endpoints used in clinical trials to evaluate the efficacy of new developmental therapies should be associated with a clinically meaningful outcome. To confirm surrogacy in patients treated with new versus standard interventions, there must be a strong correlation between the relative effect of the treatment on the surrogate and the relative effect on the true endpoint (Collette 2008; Collette et al. 2006; Johnson et al. 2003).
In recent years, biochemical recurrence by itself as well as PSA kinetics have been analyzed as surrogate endpoints to predict of prostate treatment outcomes in clinical studies. Most commonly, the “Prentice Criteria” have been used to demonstrate the validity of a putative surrogate endpoint (e. g. PSA) as a replacement endpoint for a true endpoint (e. g. survival). The Prentice Criteria require four conditions to be fulfilled (Prentice 1989):
(a)
There must be a statistically significant treatment effect on the PSA endpoint (in univariate analysis)
(b)
There must be a statistically significant treatment effect on survival (univariate analysis)
(c)
The PSA endpoint must be a statistically significant prognostic factor for survival (univariate analysis)
(d)
The treatment effect on survival must completely vanish in a survival model with both the treatment and the PSA endpoint as explanatory variables (multivariate analysis).
More recently, a methodology known as the “meta-analytic validation” has been developed. This method consists in deriving a model to predict in new trials the treatment effect on survival from the observed treatment effect on the PSA end point using survival and PSA data from several randomized trials (Collette 2008; Collette et al. 2006).
One of the first studies addressing the surrogacy issue was reported by D’Amico and colleagues in 2003 who studied PSA doubling time as a potential surrogate for prostate cancer mortality in a non-randomized cohort of 5918 men treated with surgery and 2751 with radiation (D’Amico et al. 2003). They showed that the Prentice criteria were fulfilled when using PSA doubling time less than 3 months. However, the applicability of the results was limited by the use of data from a community treatment outcomes database, which were not collected prospectively and the fact that only few patients actually had a PSA doubling time shorter than 3 months. Nevertheless, PSA doubling time at a cutpoint of less than 3 months emerged as a promising surrogate endpoint candidate for prostate cancer-specific mortality, and the place of PSA doubling time in future surrogacy analyses was established.
Using data from the RTOG trial 92-02 that compared short-term versus long-term androgen deprivation in addition to irradiation for T2c-T4 prostate cancer, Sandler and colleagues showed that time to biochemical recurrence (defined according to the ASTRO definition) was not a surrogate for cancer-specific survival (Sandler et al. 2003). Time to PSA failure was longer in the long-term androgen deprivation arm but the survival time after PSA failure was shorter. This was thought to be due to the finding that secondary therapy was more successful in men failing short-term androgen deprivation who survived longer than men who failed long-term androgen deprivation.
Valicenti and colleagues reported from the same study that post-treatment PSA doubling times of less than 6, 9, and 12 months were statistically significantly associated with prostate cancer—specific survival, but did not meet all of Prentice’s requirements for a surrogate endpoint of cancer-specific survival. Thus, the authors concluded that the risk of dying of prostate cancer cannot fully be explained by PSA doubling time (Valicenti et al. 2006).
Denham and colleagues studied the value of both PSA doubling time and time to biochemical failure (defined according to the Phoenix criteria) as surrogate candidates for prostate cancer-specific mortality after primary therapy using data from the Trans-Tasman Radiation Oncology Group (TROG) trial 96.01 (Denham et al. 2008). In this trial, 802 patients receiving radiotherapy for locally advanced, nonmetastatic prostate cancer were randomly assigned to 0, 3, or 6 months neoadjuvant short-term androgen deprivation. The authors reported that all four Prentice criteria were fulfilled and that prostate cancer-specific mortality was successfully predicted at all time to biochemical failure cutpoints between 1.5 and 2.5 years, and by PSA doubling time at the less than 12 and less than 15- month cutpoints. Best fits to prostate cancer-specific mortality were provided by time to biochemical failure at the less than 1.5 and less than 2 year cutpoints and at the PSA doubling time cutpoint of less than 12 months. The predictive value of the surrogate endpoints, time to biochemical failure less than 2 years and PSA doubling time less than 12 months, is shown in the cancer-specific survival plots (Fig. 3). The authors concluded that time to biochemical failure and PSA doubling time were prognostic variables potent enough to act as surrogate endpoint candidates for prostate cancer-specific mortality.
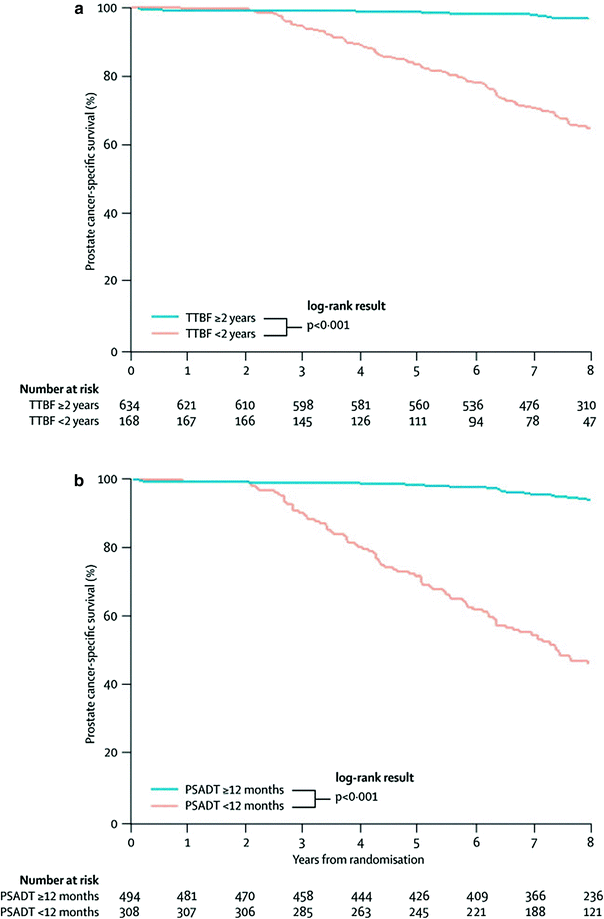

Fig. 3
Time to prostate cancer death from randomisation, stratified by a time to biochemical failure (TTBF; <2 vs ≥2 years) and b prostate-specific antigen doubling time (PSADT; <12 months vs ≥12 months); adopted from Denham et al. (Denham et al. 2008)
In the same dataset, Denham and colleagues also determined how the occurrence of biochemical failure itself related to prostate cancer-specific mortality and demonstrated that the occurrence of biochemical failure was a far weaker predictor of cancer death than time to biochemical failure (Denham et al. 2009). It was also shown that prognostic factors for early cancer death changed dramatically at biochemical recurrence. Highly prognostic factors at randomization, such as high Gleason score grouping and T classification, lost their prognostic importance, whereas factors such as shorter time to biochemical failure and PSA doubling time became powerful predictors of prostate cancer death at this time point.
In two meta-analytic validations using data from the bicalutamide trials, biochemical progression-free survival as surrogate for clinical progression-free survival in nonmetastatic disease and for overall survival in metastatic disease was studied. In patients with metastatic disease, two definitions of time to PSA progression (TTPP) were assessed: (1) For TTPP-1, PSA progression was defined as a PSA value above normal (4 ng/mL), representing a first increase 20 % above the nadir. (2) For TTPP-2, PSA progression was defined as a PSA value 2.5 times the normal range (10 ng/mL), representing a first increase 50 % above the moving average (based on three consecutive measurements) nadir. In the analyses, only a moderate correlation between the treatment effects on the PSA end point and on the clinical end point was observed. The authors concluded that a treatment benefit for the PSA end point is not sufficient to guarantee a benefit for the clinical end point but may help in decision making to prematurely stop a phase 3 trial, as in the absence of a clear benefit for the PSA end point, any benefit on clinical relapse or survival is very unlikely (Collette et al. 2005, 2006; Newling et al. 2004).
D’Amico and colleagues assessed whether two metrics of PSA (PSA value after completion of therapy (PSA end) and PSA nadir) could act as surrogates for prostate cancer-specific mortality (D’Amico et al. 2012). Their analysis included individual patients’ data from two randomized controlled trials—the Dana Farber Cancer Institute (DFCI) 95-096 trial (D’Amico et al. 2008) and the TROG 9601 trial (Denham et al. 2008) that showed a statistically and clinically significant reduction in prostate cancer-specific mortality when 6 months of androgen suppression was added to radiotherapy compared to radiotherapy alone. The cutoff values were chosen on the basis of reports suggesting that a PSA nadir of more than 0.5 ng/mL after radiotherapy and short-course androgen suppression was associated with an increased risk of recurrence (Benchikh El Fegoun et al. 2008; Lamb et al. 2011; Zelefsky et al. 1998). The authors found that both PSA nadir and PSA end concentrations of more than 0.5 ng/mL were surrogates for prostate cancer-specific mortality, permitting early identification of men in whom radiotherapy and 6 months of androgen suppression is insufficient for cure. The authors suggested that men with PSA end values exceeding 0.5 ng/mL following radiotherapy and 6 months of androgen suppression should be considered for long-term androgen suppression. Additionally, clinical researchers could use the PSA nadir of more than 0.5 ng/mL as an eligibility criteria for an early (at time of PSA nadir) versus delayed (at time of PSA failure) intervention with salvage androgen suppression (D’Amico et al. 2012).
5 Conclusion
PSA is an important tool for monitoring disease progression following treatment of definitive localized prostate cancer allowing for early measure of treatment failure, long before clinical disease becomes evident or survival is affected.
However, a consensus on the ability of biochemical failure to act as a surrogate endpoint for survival has not yet been reached. Although most agree that biochemical failure is a useful surrogate for treatment efficacy, the relationship and timeline between PSA failure, clinical failure, and survival is less well documented.
The value of biochemical recurrence by itself as a predictor of prostate cancer-specific mortality has been questioned lately because survival after biochemical recurrence is highly variable. Thus, in recent years an interest in analyzing the PSA kinetics has been generated to help refine the ability to predict of prostate treatment outcomes. Several studies to identify a PSA endpoint as a surrogate endpoint for prostate cancer-specific survival have focused on the kinetics of increasing post-treatment serum PSA levels and have found that rapid PSA increases, or short post-treatment PSA doubling times, after local therapy are associated with the development of metastatic disease and prostate cancer—specific mortality.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree