Abstract
Background
Despite early treatment of rheumatoid arthritis (RA) being well established to prevent erosive joint damage, studies report persistent moderate to high disease activity. Other pathologies, for example, rheumatoid vasculitis (RV) may contribute symptoms that may not be captured by existing clinical assessment.
Objectives
To investigate ultrasound-observed changes in the proximal dorsalis pedis artery (DPA) between early (≤ 5 y) and established (>5 y) RA and the RA Disease Activity Index-5 (RADAI-5).
Methods
Participants with early ( n = 20) and established RA ( n = 20) were recruited. Five parameters of the DPA were examined with a previously established ultrasound method. Independent t-tests and Cohen d statistics assessed differences and effect size between ultrasound parameters and RADAI-5, and the two groups. Pearson correlation assessed associations between ultrasound parameters and RADAI-5.
Results
Majority of participants (98%) demonstrated arterial wall thickening regardless of disease duration. However, lumen diameter (Cohen’s d = 0.972, p = 0.004) and artery diameter (Cohen’s d = 0.694, p = 0.034) were decreased in established RA compared to early RA. No strong associations were found between RADAI-5 and ultrasound parameters, except for lumen diameter in early RA demonstrating a fair association to RADAI-5 ( r = 0.445). The mean RADAI-5 score indicated moderate to high disease activity with no difference between early and established RA ( p = 0.283).
Conclusion
Arterial wall thickening of the DPA indicating the precursory changes of RV was observed in most RA participants, with reductions in artery and lumen diameter occurring in established disease. However, the long-standing instrument RADAI-5 may not reflect symptoms and clinical impacts related to vascular changes among people with RA.
Introduction
Rheumatoid arthritis (RA) is an autoimmune disease characterized by well-established clinical and imaging changes, such as synovitis and joint damage [ , ]. Diagnosis of RA is based upon symmetrical painful joint stiffness from disruption to the synovium and inflammatory markers present within the blood [ ]. However, clinical diagnostic challenges exist [ ] with up to 50% of patients being seronegative [ ]. Disease management protocol outlines early RA disease as approximately the first 6 y from diagnosis and therefore initiation of treatment is preferred within this timeframe [ , ], with the optimal window of treatment considered in the first few months [ , ]. Disease progression timelines from early to established disease are also evolving, made more difficult when synovial changes can remain constant across early and established disease indicating active disease [ ]. This may explain, that despite current best management protocols, self-reported moderate to high disease activity levels are still reported, using tools such as RA Disease Activity Index-5 (RADAI-5) [ ]. Furthermore, extra-articular comorbidities such as rheumatoid vasculitis (RV) may also play a part in persistent moderate to high disease activity reports.
RV, a systemic connective tissue vascular disease [ ], may contribute to multiple pathologies including dermatological, such as digital ischemia and non-healing ulcers (90%), peripheral neuropathy (40%) and cardiac disease (30%) with serious health consequences [ ]. Rather than a latter presentation in established RA disease, recent research indicates RV coexists with RA early in the disease process [ ], when management would be most beneficial [ ]. However, diagnosis remains challenging [ ] indicated by sudden cardiac death risk being 50 to 70% higher in patients with RA compared to the general population [ ]. Additionally interstitial lung disease is also present in up to 60% of patients with RA [ , ]. These comorbidities contribute to the increased morbidity and mortality for patients with RA [ ].
Recent advances in medical imaging have expanded our understanding of vascular pathology in rheumatic diseases. Ultrasound identification of the halo sign, characterized by arterial wall thickening, has proven diagnostic for giant cell arteritis when combined with high clinical suspicion [ ]. Similarly, modified ultrasound techniques have revealed significant differences in dorsalis pedis artery (DPA) wall thickness between individuals with and without RA, suggesting precursory changes of RV [ ]. Intriguingly, these vascular alterations may not be captured by traditional measures such as ankle-brachial index (ABI) [ ] or peak systolic velocity (PSV) [ ], highlighting the unique nature of vasculitis-associated arterial changes and the potential limitations of conventional diagnostic approaches.
For example, in Behcet’s disease which can present as subclinical vasculitis, ABI results are normal, thus suggesting no changes in arterial wall resistance [ ]. Furthermore, when people with Behcet’s, RA and healthy controls were compared, no statistical differences in ABI were also found [ ]. This is in contrast to atherosclerotic disease, where increased arterial wall resistance results in abnormal ABI [ ]. Importantly in atherosclerotic disease, abnormal ABI and associated increased PSV and stenotic lesions can be confirmed by medical imaging techniques [ ]. However, unlike vasculitis, without abnormal PSV or ABI, further imaging or clinical assessment may not proceed. Additionally, complications from stenting may be particularly high in the presence of vasculitis associated extended arterial wall thickening [ ]. This also explains why stenting is not recommended as first-line treatment for vasculitis despite progressive chronic lower limb ischemia [ ].
Despite recent findings of arterial wall thickening indicative of the precursory changes of RV [ ], and its association with declining foot health status in RA [ ], there remains a critical gap in our understanding of how these vascular changes evolve across the disease spectrum. Our study aims to address this knowledge gap by investigating arterial changes in the proximal DPA between early and established RA and exploring their relationship with disease activity as measured by the RADAI-5.
We hypothesize that individuals with established RA will exhibit more pronounced arterial changes indicative of precursory RV within the DPA. Furthermore, we suggest that while the RADAI-5 is a validated assessment tool for RA, it may not fully capture the subtle vascular changes occurring in the DPA. This investigation seeks to shed light on the temporal progression of vascular involvement in RA and evaluate the sensitivity of current disease activity measures to these important vascular alterations.
Method
Participants
Participants with a medical diagnosis of RA were recruited from the public, via colleagues, community groups and social media. Eligible participants provided written informed consent, under the approved human ethics conditions from the Human Ethics Committee, Western Sydney University (Reference number: H14696) and attended one appointment of approximately 1 h. People who smoked, had diabetes or arterial leg surgery were excluded as they are these factors are known contributors to peripheral arterial disease, therefore reducing overlap between atherosclerosis and vasculitis.
All participants had a medical diagnosis of RA from a rheumatologist and this diagnosis was the marker for day of diagnosis. Participants were categorized into two disease duration groups, 5 y or less and greater than 5 y, given the pivotal point for early treatment is recommended within the first 5 y of diagnosis [ , , ].
Experimental setup
The participants were in a temperature-controlled room (i.e., 24–26°C) while in a semi-reclined restful position. Mindray DC40, M8 and M9 ultrasound machines (Mindray, Medical International Limited, Shenzhen, China), were used with linear transducers (L13-3, L12-3 and L10-3, respectively). To visualize the soft tissue changes of the DPA wall, a musculoskeletal preset was selected which provided a wider dynamic range to assess soft tissue changes [ ]. The five parameters were wall thickening, lumen diameter, arterial diameter, lumen-to-artery diameter ratio and PSV with a detailed imaging protocol described previously [ ]. Four b-mode parameters of the proximal DPA, have reported excellent reliability (ICC > 0.9), with PSV demonstrating more variable but acceptable reliability [ ].
Participants completed a demographic survey and the RADAI-5 [ ]. The RADAI-5 is a reliable and validated patient-reported outcome measure designed to monitor treatment in clinical practice [ ], using five questions about pain, joint symptoms and current general health with an overall score out of 10. Disease activity is rated as, in remission (0–1.4), mild (1.6–3), moderate (3.2–5.4) and high (5.6–10) [ ].
Data collection
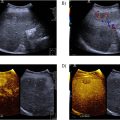
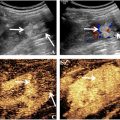
The proximal DPA was defined to start midway between the lateral and medial malleoli which is approximately at the level of the tibial mortice [ , ]. To consider normal anatomical variations of the proximal DPA and to observe the largest portion of arterial wall thickening within the proximal DPA, imaging of the DPA was performed in the longitudinal plane [ ]. Arterial wall thickening when present was measured at its thickest location, either at the anterior or posterior aspect ( Fig. 1 ). Artery diameter was measured from the external aspects of the adventitia across the width of the artery and lumen diameter from the internal aspect of the intima ( Fig. 2 ). The ratio was calculated from these aforementioned measurements. PSV was measured between the region where arterial wall thickening region was at its maximum ( Fig. 3 ). Bilateral DPA assessment was conducted with the random selection of one side used for data analysis. B-mode and Doppler imaging was utilized and aligned with the ALARA effect (as low as reasonably achievable) [ ]. Medication data was collected within the demographic survey and tabulated to describe the sample.



Statistical analysis
The data was analyzed using SPSS (Version 29.0, IBM Corp, Armonk, NY, USA). Chi-square test was employed to compare gender, and independent t-tests were employed to compare age and body mass index (BMI) between two groups of participants. Independent t-tests were used to examine the between-group differences in the selected parameters. To minimize overreliance to the p value interpretations, Cohen’s d was used to represent effect sizes of the comparisons. Cohen’s d effect sizes are interpreted as small (0.2), medium (0.5) and large effect (0.8) [ ]. Pearson correlation was used to assess relationships between RADAI-5 scores and the five ultrasound parameters. The strength of Pearson correlation is described as none (0), poor (0.1 to 0.2), fair (0.3 to 0.5), moderate (0.6–0.7), very strong (0.8–0.9) or perfect (1.0) [ ].
Results
Forty participants with medically diagnosed RA participated in this study, with 20 participants with disease duration of 5 y or less and 20 participants with greater than 5 y. The two subgroups demonstrated no statistical difference for gender ( χ 2 (1), p = 1.00); age ( t (38) = −1.655, p = 0.106) and BMI ( t (38) = 0.526, p = 0.602). Similar RA medication profiles (single or multiple prescriptions) of prednisolone (glucocorticoids), non-steroidal anti-inflammatories (NSAIDS), disease-modifying antirheumatic drugs, biological agents and Janus Kinase inhibitors were reported between groups ( Table 1 ). Overall, there was more observed RA medication prescriptions in established disease, with the exception of NSAIDS with much greater use ( Table 1 ). A further observation was participants reported similar co-morbidities such as hypertension and hypercholesterolemia ( Table 1 ).
| Characteristics | RA Disease Duration | p | ||
|---|---|---|---|---|
| ≤5 Y ( n = 20) | >5 Y ( n = 20) | |||
| Gender (female/male) | 18/2 | 18/2 | 1.000 | |
| Age (y) | 54.9 ± 10.9 | 60.6 ± 10.9 | 0.106 | |
| BMI (kg/m 2 ) | 30.5 ± 7.5 | 29.4 ± 5.2 | 0.602 | |
| RADAI-5 score (/10) | 5.4 ± 1.8 | 4.6 ± 2.8 | 0.283 | |
| RADAI-5 levels | ||||
| Stable/remission | (0.0–1.4) | 0 | 4 | |
| Mild | (1.6–3.0) | 1 | 2 | |
| Moderate | (3.2–5.4) | 10 | 6 | |
| High | (5.5–10.0) | 9 | 8 | |
| RA medications | ||||
| JAK inhibitor | 3 | 4 | ||
| Biologics | 4 | 6 | ||
| DMARD | 9 | 12 | ||
| NSAID | 1 | 11 | ||
| Prednisolone | 2 | 5 | ||
| Other medical history | ||||
| Hormone replacement therapy | 1 | 4 | ||
| Hypertension | 6 | 7 | ||
| Hypercholesterolemia | 3 | 5 | ||
| Depression | 2 | 3 | ||
| Osteoarthritis | 6 | 6 | ||
| Back pain | 6 | 8 | ||
| Heart disease | 1 | 0 | ||
| Lung disease | 1 | 0 | ||
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree