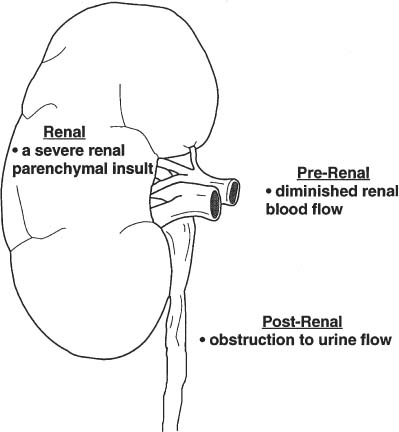
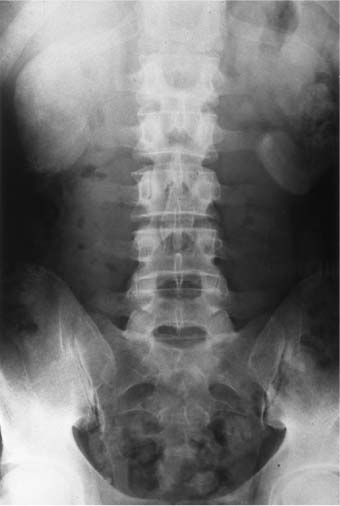
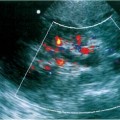
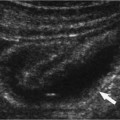
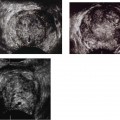
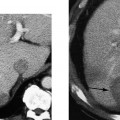
2 Renal Failure Renal failure refers to inadequate renal function with the accumulation of nitrogen waste to a toxic level that is incompatible with life. A distinction is made between renal failure and renal insufficiency. Renal insufficiency is the abnormal accumulation of nitrogen waste with preservation of sufficient renal function to sustain life. End-stage renal disease is chronic and irreversible renal failure; renal dialysis or transplantation is necessary for survival. The prevalence of renal failure is ~65 cases per million in the United States. Less than 20% of acute renal failure patients eventually require dialysis. At autopsy, ~25% of uremic patients have obstructive uropathy as either a contributory or major cause of renal insufficiency.1 Renal dysfunction, whether it be insufficiency or failure, produces the clinical state of uremia. Renal failure is the final common pathway for numerous disparate disease processes affecting the kidney. During the initial clinical evaluation, it is essential to quickly determine if renal failure is reversible. Thus determining the etiology is crucial to identify reversible causes of renal dysfunction. Diagnosis depends on evaluation and integration of the clinical history, physical exam, and laboratory data. Maintenance of the normal internal milieu of the body depends on the ultrafiltration of blood by the kidney, followed by the secretory and resorptive functions of renal tubules. Renal function leads to the excretion of urine via the ureters, bladder, and urethra. Based on the physiology of renal function, renal failure is classified as pre-renal when caused by diminished renal blood flow; intrinsic when caused by renal parenchymal damage; and postrenal when caused by blockage of urine flow (Fig. 2–1). For the referring clinician and radiologist, the initial step in assessing renal failure is to categorize the etiologic event as prerenal, renal, or postrenal. The ability to intervene and provide prompt improvement in defective renal function is limited to prerenal and postrenal processes. Although intrinsic renal processes may be ameliorated, the possibility of an immediate cure is unlikely. Diagnosis of prerenal failure focuses on levels of the blood urea nitrogen (BUN) and serum creatinine. Both BUN and creatinine are filtered freely at the glomerulus. Normally, urea, but not creatinine, is reabsorbed at the tubules in peritubular capillaries. With decreased renal blood flow, capillary flow decreases and more BUN is resorbed. The resulting disproportionate elevation of blood levels of BUN compared with creatinine, can be used as a diagnostic indicator of prerenal renal dysfunction related to renal blood flow decrease. Postrenal acute renal failure has a variable clinical presentation depending on the site and completeness of the obstruction. Urine volumes in the setting of obstruction are quite variable. Reduced urine volume and azotemia usually develop only when the obstruction is located at the level of the bladder or below. Azotemia from ureteral obstruction develops only when the obstruction is bilateral or there is a solitary kidney. Obstruction is usually reversible. When the kidney becomes obstructed, renal function changes with time. Paralleling the alteration in renal function is an alteration of the composition of urine as a result of impaired water reabsorption and electrolyte transport. Intrinsic disorders affecting the renal parenchyma cause decreased glomerular filtration rate (GFR) and resultant elevation of the BUN and creatinine. There is a difference between hospital-acquired and non-hospital-acquired intrinsic (acute) renal failure. Most hospital-acquired acute renal failure is caused by acute tubular necrosis (ATN). Intrinsic acute renal failure acquired outside the hospital is usually caused by acute glomerular, interstitial, or vascular disease. When prerenal and postrenal acute renal failure are excluded, the cause of acute renal failure must be an acute renal parenchymal insult. Figure 2–1 The three main types of renal failure are based on an etiologic classification. The clinical difficulty in diagnosing the cause of acute renal failure arises from the plethora of potential etiologies and the difficulty distinguishing among them. It is often difficult or impossible to determine if the cause is prerenal, intrinsic, or postrenal. Compounding the diagnostic confusion is the possibility that several different causes of renal dysfunction may interact to produce acute renal failure. Prerenal acute renal failure should be considered if there is a history of congestive heart failure or hypotension. Changes in the patient’s weight can indicate fluid shifts caused by hypoperfusion, either from dehydration or “third spacing” of fluid. Physical examination to assess skin turgor, edema, blood pressure, and pulse is helpful. The referring clinician will usually be able to diagnose or exclude prerenal acute renal failure without imaging assistance. Postrenal acute renal failure due to obstruction is easily corrected if promptly diagnosed. For this reason, it is important to consider the possibility of obstruction as a cause of renal failure in every patient. Sonography can usually diagnose obstruction quickly and simply. It is important to evaluate the clinical history because the presence of a single kidney or preexisting renal disease will alter the presentation. A history of stone disease or malignancy suggests possible causes of obstruction. Urine volume is a nonspecific test for acute renal failure. Anuria, defined as a 24-hour volume of 100 mL or less, may occur with prerenal, obstructive, and intrinsic renal disease. The urine-specific gravity or osmolality is measured to assess the concentration of solute in the urine. With hypovolemia, water is reabsorbed, and the urine becomes concentrated. With urine osmolalities below 350 mOsm, intrinsic renal disease is most likely. With osmolalities above 500 mOsm, the likely etiology is prerenal acute renal failure. There is great overlap, however, between the range of 350 and 500 mOsm. The normal specific gravity of urine is 1.002 to 1.028. Creatinine is formed from the breakdown of muscle creatinine phosphate. Daily production is proportional to muscle mass. With acute renal failure, creatinine can rise 1 to 2 mg per dL per day. The normal range of creatinine in females is ~0.6 to 1 mg/dL and in males 0.8 to 1.3 mg/dL. Plasma levels of urea may also be used to assess renal function. Blood urea levels, unlike serum creatinine values, are commonly influenced by external factors; for example, an increased dietary load of protein or intestinal bleeding increases the plasma urea levels. Normal BUN is 7 to 18 mg/dL and may rise 10 to 25 mg/dL per day in acute renal failure. Renal biopsies should be performed in only a small subset of patients with intrinsic renal disease. In most situations, the diagnosis and likely etiology of intrinsic renal disease is sufficiently certain with clinical history, urinalysis, and physical exam that appropriate treatment can be instituted. Less than 20% of patients with intrinsic renal disease require a kidney biopsy to establish the cause of acute renal failure. A plain film of the abdomen can be useful in assessing renal size and shape and identifying radiopaque stones and renal calcification (Fig. 2–2). Normal renal size is 3.7 ± 0.37 × the height of the second lumbar vertebral body. Approximately 90% of renal stones are radiopaque. Thus, potentially obstructing stones can often be identified on plain radiographs. Renal osteodystrophy, a complication of chronic renal failure, or metastatic bone disease, may also be identified on the plain film. Figure 2–2 Plain radiograph of the abdomen in a patient with ARF demonstrates diffuse bilateral cortical nephrocalcinosis. Excretory urography is usually not performed in patients with acute renal failure because of the risks that contrast material may exacerbate renal failure or cause a hyper-sensitivity reaction. The utility of renal sonography has also contributed to decreased demand for excretory urography. When renal function is normal, 25 mg of iodine per kg body weight is appropriate for the excretory urogram. With acute renal failure, 40 to 60 mg of iodine per kg is necessary to achieve opacification of the collecting system. The urogram can provide information regarding the size and shape of the kidneys. With severe azotemia, tomography is necessary to assess the renal contour (Fig. 2–3
Differential Diagnosis
Diagnostic Evaluation
Nonimaging Evaluation
Imaging other than Ultrasound
Plain Film
Excretory Urography
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree