Pediatric Renal Transplantation
The incidence of end-stage renal failure in children under the age of 15 years is estimated to be 5-8 per million children, and is commoner in males by nearly two to one.1 The incidence in adolescents between the ages of 15 and 18 years is more difficult to determine because of referral practices, but is thought to be higher.1
Etiology of Chronic Renal Failure
The causes of renal failure in childhood are very different from those in the adult population and are outlined in Table 5.1. The majority of patients, particularly younger patients, suffer from congenital structural anomalies of the urinary tract. These conditions are often complicated by poor urinary concentrating ability and polyuria. Commonly, bladder function as well as kidney function is abnormal, and the management of end-stage renal failure must also involve the investigation and treatment of abnormal bladder function prior to transplantation.
Management of Chronic Renal Failure
For most children with chronic renal failure, progression to end-stage renal failure is a slow process. Conservative management is aimed at maintaining fluid, electrolyte, and acid-base balance, controlling blood pressure, providing an adequate calorie intake, and preventing anemia and renal osteodystrophy. This can be difficult in uremia, as a greater calorie requirement is needed to achieve normal weight gain.2 This last, coupled with anorexia and vomiting due to abnormal gut motility, compounds the problem. In some children this is further aggravated by excessive thirst due to marked polyuria. Often an enteral feed, either with a nasogastric tube or with a gastrostomy tube, is the only way of ensuring a sufficient calorie intake.
Children with oligoanuria will need fluid restriction, but many children with chronic renal failure pass significant quantities of urine. The enteral route allows the provision of adequate fluid and electrolyte supplements and can correct metabolic acidosis. Anemia of chronic renal failure can be corrected with recombinant human erythropoietin once sufficient iron stores have been achieved, and activated vitamin D and phosphate restriction prevent renal osteodystrophy.
| Renal dysplasia and related conditions | 28 |
| Obstructive uropathy | 20 |
| Glomerular disease | 17 |
| Reflux nephropathy and chronic renal failure of uncertain etiology | 9 |
| Primary tubular and interstitial disorders | 7 |
| Congenital nephrotic syndrome | 7 |
| Renal vascular disorders | 5 |
| Metabolic diseases | 3 |
| Polycystic disease | 2 |
| Malignant and related disorders | 2 |
Peritoneal Dialysis
Peritoneal dialysis is the treatment modality of choice in childhood. Although less efficient than hemodialysis, it is better tolerated in children. Furthermore, automated peritoneal dialysis can be carried out overnight at home, with little disruption to schooling. Even very small infants can tolerate peritoneal dialysis, but it is a huge undertaking to dialyze a baby from birth, and it is a treatment which can be fraught with complications. In general, the success of peritoneal dialysis in small infants is related to the presence of residual renal function and the lack of nonrenal co-morbid factors.3 The main complications of peritoneal dialysis are exit site infections and peritonitis, particularly in infants and children who are still in diapers. The presence of a coexisting gastrostomy tube does not increase the incidence of peritonitis, but insertion of a gastrostomy tube once dialysis has been established does increase the risk of peritonitis.4 Prolonged periods of time on peritoneal dialysis can result in membrane failure or, more rarely, sclerosing peritonitis.
Hemodialysis
In patients who have undergone extensive abdominal surgery, or those who have suffered from peritoneal membrane failure, hemodialysis is required. In all but the older child, double-lumen indwelling cannulae are used. Hemodialysis is more disruptive in terms of schooling, and may be poorly tolerated in the younger child, in whom achievement of fluid balance is more difficult.
Transplantation
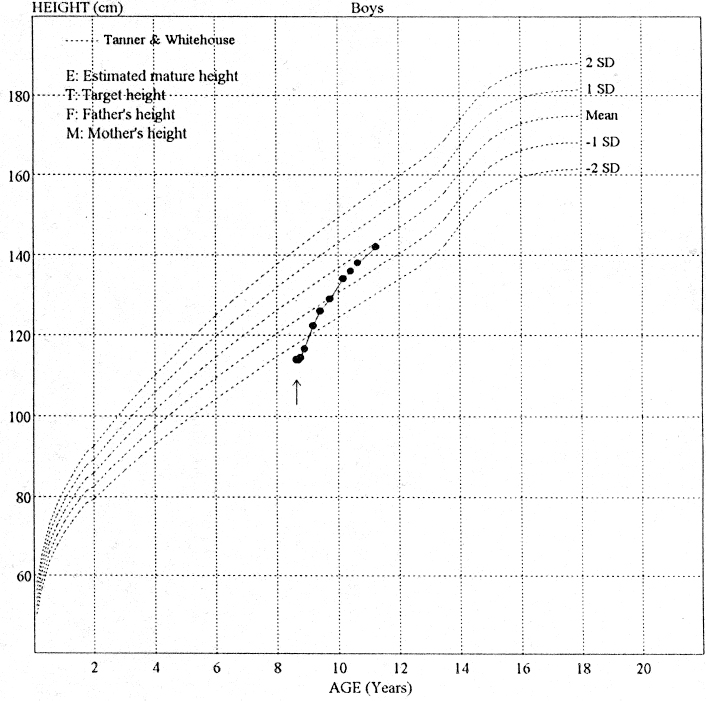
In addition to offering more effective renal replacement therapy, transplantation offers better physical and psychological well-being and social rehabilitation. In the case of children this includes better growth and nutrition (Fig. 5.1), and in infants better neurological development.5 Following transplantation, children are more likely to have a better attendance record at school and to partake in normal childhood activities.6 Increasingly, children are receiving transplants without first undergoing dialysis (so-called pre-emptive transplantation), which avoids disruption to schooling, periods of ill health on dialysis, and poor growth.6 Graft survival data for pediatric patients are now as good as for adults; three-year graft survival for both pediatric and adult cadaveric transplant recipients is 77%.7
Living Related Donation
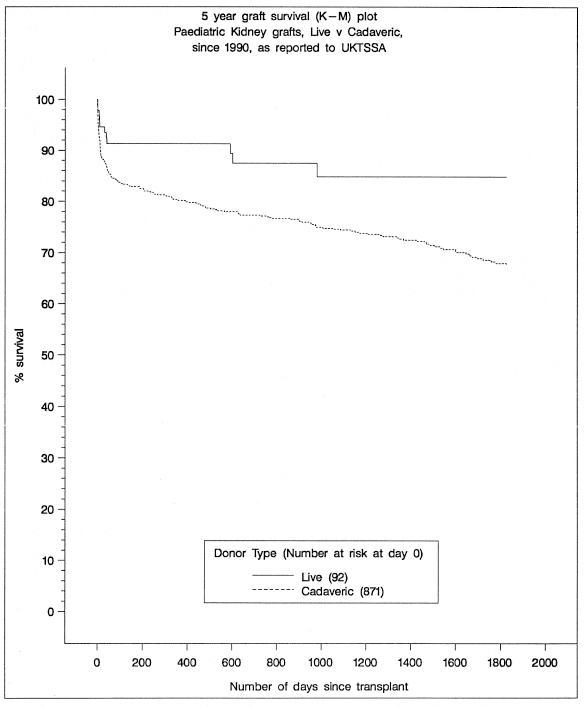
The decision as to cadaveric versus living related donor transplantation depends on the individual patient’s circumstances. The results for living related donor kidneys are superior to those of cadaveric transplantation at all ages (Fig. 5.2), but the difference is most marked in young recipients.8,9 Living related donation generally involves parents, but grandparents, aunts, uncles, and older siblings can all be considered. Living unrelated donation can be performed with adopted parents.
The incidence of living related donation varies from country to country. In the United Kingdom the figure is 16 %,10 whereas in North America 49% of pediatric transplants are from living donors,9 and in Scandinavia the figure is as high as 86%.11 The variation is due to differences in organ-sharing programs and to geographical and cultural factors.
For many families, an advantage of living related donation is that it allows a degree of control over the timing of surgery. It also allows all required personnel to be present at the operation and both child and donor to be in the best possible health. Other advantages of living related donation are that preemptive transplantation is more easily performed, the incidence of acute tubular necrosis is reduced,12 and graft survival is improved.12,13 The disadvantage of living related donation is that one of the child’s carers becomes a patient, leaving the other carer with two dependants to look after in addition to any other siblings.
Preemptive Transplantation
The decision to start renal replacement therapy depends upon biochemical factors as well as clinical parameters. Failure of conservative management to control acidosis, hyperkalemia, or fluid overload, as well as failing growth, failure to thrive, and the inability to manage a full day at school are taken into account. In general at this stage, the glomerular filtration rate is between 5 and 10 ml/min per 1.73 m2.14 Living related donation allows greater control over the timing of transplantation, but cadaveric preemptive transplantation is also possible.
At present between 20 and 25% of transplants are performed preemptively.14,15 Children who go directly to transplantation are generally healthier, and many of them have no experience of inpatient admission. The perioperative time can therefore be a very difficult time. Concern has been expressed that preemptive transplantation does not allow patients to experience how unwell they can feel on dialysis and therefore appreciate the benefits of transplantation. There is no evidence in pediatric practice that this is the case, however14 ; indeed there is preliminary evidence that preemptive transplantation has a beneficial effect, with improved graft survival in living donor recipients.15
The Young Recipient
Infants with chronic renal failure often have developmental delay as well as failure to thrive. Dialysis does little to improve development and growth in infants,16 but following transplantation impressive catch-up growth is seen,17 and, more importantly, there is an improvement in the indices of mental and motor development.5
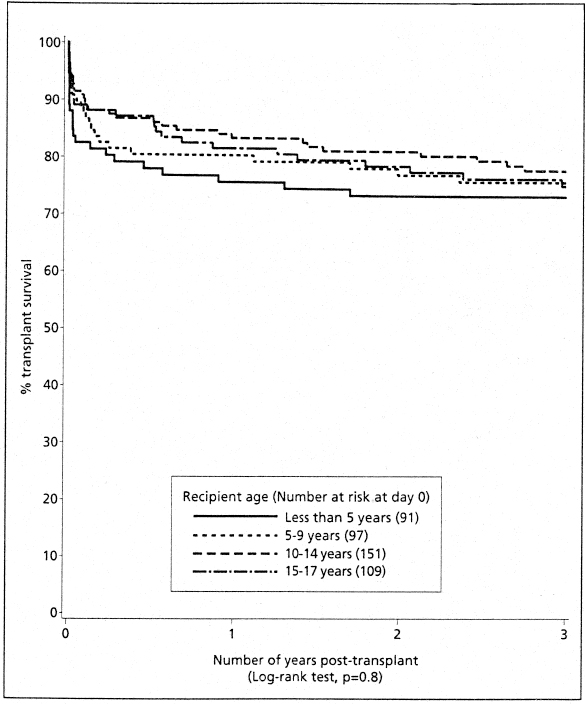
Early results of transplantation in young recipients were poor, especially for cadaveric grafts.12 Vascular thrombosis and technical difficulties are important causes of graft loss in the under-5 age group.18–20 At this age the immune system is more active,21 and there is increased metabolism and more variable absorption of cyclosporine.22 However, there have been improvements in graft survival in young recipients (Fig. 5.3) related to the use of more living related donors and the avoidance of young donor organs.8,19,23 Excellent long-term graft survival and graft function have been described in this age group,24 which is no different to those seen in older recipients.25 Within the under-5-year age group, results for the under-2-year-olds do not differ from those for children over 2 years,19,25,26 although transplantation of the very young recipient is best carried out in specialized centers. Fluid management is critical in this group, particularly when the donor organ is relatively large in proportion to the recipient. In this situation elective postoperative ventilation aids management. The use of a living donor organ provides the best option25; a body weight of 7-8 kg has been suggested as the minimum necessary for receiving an adult-sized kidney.26
 Summary points:
Summary points:
- More likely to have underlying structural anomalies of the renal tract
- More likely to receive a larger donor kidney
- Operative fluid management critical
- More likely to have acute tubular necrosis
- Higher incidence of early graft loss
Contraindications to Transplantation
In contrast to adults, the majority of children with end-stage renal failure will be suitable for transplantation; contraindications are the presence of malignancy within the previous two years, HIV infection, and elevated circulating anti-glomerular basement membrane antibodies. Renal transplantation is not carried out in the presence of ABO blood group incompatibility, nor in the presence of cytotoxic antibodies against donor antigens. Consideration needs to be given to the quality of life following transplantation, therefore multiple organ failure and severe brain damage will in general preclude transplantation. Transplantation should be delayed for several months if there is ongoing active disease such as hemolytic uremic syndrome or rapidly progressive glomerulonephritis. Patients with hepatitis B or C can proceed with transplantation in the absence of active liver involvement.
Work-Up for Transplantation
To optimize the chances of success, it is important to investigate the child thoroughly before transplantation. Several areas require attention.
Sensitization
Anti-HLA antibodies may be formed during blood transfusions or as a result of a previous graft. These are screened for prior to transplantation, and if any are detected, the relevant antigen should be avoided in the donor.
Viral Infections
It is also important to screen for prior exposure to viral infections (cytomegalovirus [CMV], Epstein-Barr virus [EBV], varicella, hepatitis B, and hepatitis C). Antiviral prophylaxis is given to CMV-seronegative recipients who receive CMV-positive grafts, and varicella vaccination is performed in those children who have not been exposed to varicella.
Urinary Tract
Imaging of the native kidneys and bladder should be performed (Table 5.2). It is important to determine whether the child can empty the bladder completely as residual urine is a risk factor for urinary tract infection, as is the presence of marked reflux into large dilated ureters. Large-capacity bladders which do not empty completely may be helped with intermittent catheterization either per urethra or via a Mitrofanoff stoma. Small bladders which are under high pressure can result in damage to the transplanted kidney, and bladder augmentation may be required prior to surgery. If the bladder capacity is inadequate, a transplant ureterostomy is necessary (Fig. 5.4).
Initial imaging with ultrasound can show bladder wall thickness, ureteric abnormalities, and bladder emptying (Fig. 5.5). For those with known bladder abnormalities, a micturating cystogram will give more information on bladder capacity and reflux (Fig. 5.6). In those found to have an abnormal bladder, formal urodynamic studies should be performed. Initial results of transplantation into recipients whose renal failure was due to a posterior urethral valve were poor,27 with grafts becoming scarred from a combination of functional obstruction and infection. More recent data report similar graft survival data for bladder outlet obstruction as compared to other causes of chronic renal failure in childhood.28
Native Nephrectomy
Up to 24% of children undergo native nephrectomies prior to transplantation.12 The main indications include recurrent urinary tract infections, severe hypertension, persistent nephrotic state, and massive enlargement of polycystic kidneys causing respiratory or gastrointestinal compromise.
Vasculature
Large kidneys transplanted into small children require a greater blood flow than smaller kidneys. These grafts are placed onto the aorta and inferior vena cava rather than the iliac vessels. It is helpful to know the size and patency of the major vessels prior to transplantation.26
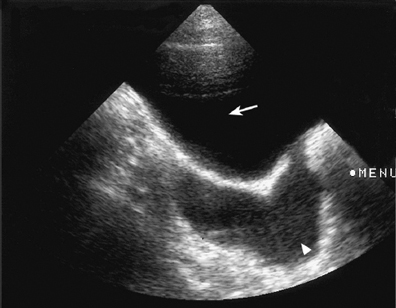
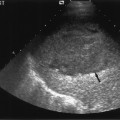
Fig. 5.5 Ultrasound scan of the bladder (arrow) showing a dilated left ureter (arrowhead) at the level of the bladder. After voiding, a significant postvoid residue was observed. A cystogram showed the presence of gross left-sided vesicoureteric reflux
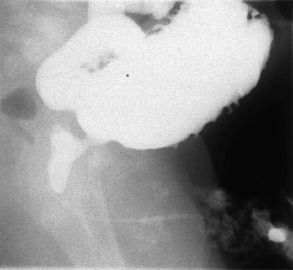
Fig. 5.6 Micturating cystourethrogram showing the presence of a posterior urethral valve with dilatation of the posterior urethra. The bladder is cone-shaped with multiple diverticula
Pretransplant:
|
Perioperative:
|
Postoperative:
|
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree