Because of a recent increase in survival rates and life expectancy of patients with congenital heart disease (CHD), radiologists are facing new challenges when imaging the peculiar anatomy of individuals with repaired CHD. Cardiac computed tomography and magnetic resonance are paramount noninvasive imaging tools that are useful in assessing patients with repaired CHD, and both techniques are increasingly performed in centers where CHD is not the main specialization. This review provides general radiologists with insight into the main issues of imaging patients with repaired CHD, and the most common findings and complications of each individual pathology and its repair.
Key points
- •
Continuous advancements in diagnostic and therapeutic techniques have led to an increased long-term survival of patients with congenital heart disease.
- •
Imaging of repaired congenital heart disease is becoming a common routine in radiology practice.
- •
General radiologists need to be familiar with the most common congenital heart disease, surgical techniques, and complications.
Introduction
Congenital heart disease (CHD) has a prevalence of 4 to 10 per 1000 live births. The median age of patients with CHD in the United States has increased from 11 to 17 years between 1985 and 2000, and continues to increase today. Since the first successful repair of CHD with cardiopulmonary bypass more than 70 years ago, survival of children with CHD has greatly improved: from an estimate of 20% of patients reaching adulthood, survival rose to 80% to 85%. Today, in the United States, there are more adults living with CHD than there are children. Adult patients with CHD present a challenge to the general radiologist because of the unique anatomy and physiology, which is often associated with their disease processes.
The overarching goal of this review article was to provide general radiologists with an insight into the main issues of imaging patients with repaired CHD, and the most common findings and complications of each individual pathology and its repair, focusing on tetralogy of Fallot (ToF), transposition of the great vessels, functional univentricular heart, aortic coarctation, anomalous pulmonary venous return, and atrial septal defects (ASDs).
Imaging techniques
Although serial electrocardiograms (ECG) and echocardiograms continue to be important first-line modalities to evaluate patients with CHD, and catheterization represents the invasive reference standard, cardiac computed tomography (CCT) and magnetic resonance (CMR) are indispensable noninvasive second-level tests for a comprehensive evaluation of such individuals. Specifically, CCT and CMR are superior to echocardiography when analyzing the right ventricle (RV), great vessels, pulmonary circulation, coronary arteries, and cardiac valves.
Computed Tomography
In more recent years, use of CCT has become increasingly prevalent for the evaluation of patients with CHD, because of advancements that allow for faster scans, with very low radiation doses. CCT is most useful in those particular cases when CMR is not feasible: for instance in the presence of CMR-unsafe devices, or in the case or claustrophobic, obese, critically ill, or unstable patients, as well as when coronary arteries, prosthetic valve integrity, or calcifications are under consideration.
It is common for CCT protocols in patients with CHD to include an ECG-synchronized unenhanced scan in case of suspected acute aortic pathology, in the evaluation of calcifications or postsurgical or degenerative modifications. Furthermore, in certain cases, the administration of contrast agent may be contraindicated. However, contrast-enhanced scans are the mainstay of CCT protocols in CHD. Iodinated contrast volumes range from 1 to 2 mL/kg in children to approximately 120 mL in adults. They should be acquired at the appropriate time after injection with regard to the structure that is the main objective of the examination.
Contrast-enhanced CCT can provide valuable morpho-functional information, including measurements of ventricular volumes and ejection fraction with a reliability comparable to that of CMR. It is the current reference standard for an in vivo evaluation of cardiac and vascular anatomy, because of its high spatial and temporal resolution. Another potential advantage of CCT is that, because examinations take less time, sedation may be needed less often than in CMR, where it is recommended for every patient younger than 8 years. This may be a significant advantage in patients with CHD, as most of them need second-level imaging studies during childhood, and they may be more prone to comorbidities or side effects secondary to sedation.
One of the major concerns associated with CCT in patients with CHD is radiation exposure, as these patients are frequently subjected to repeated examinations over the course of their lifetimes. In this view, a proper choice for image acquisition synchronization is a main issue, as prospective gating is useful for the evaluation of great vessels, whereas retrospectively ECG-gated scans may provide more detailed information, especially regarding coronary arteries, albeit with a trade-off in terms of higher radiation exposure. In this regard, scanners commonly found in clinical settings, from 64-row multidetector CCT to more recent scanners using cutting-edge technology, are able to provide reasonably low radiation doses.
Magnetic Resonance Imaging
Along with CCT, CMR is an important second-line diagnostic test used in patients with CHD: the 2018 American Heart Association guidelines for the management of adult CHD report a class I recommendation for serial CMR examinations in patients who are at risk of developing RV dysfunction.
CMR examinations in patients with CHD typically include nonenhanced stacks of cine sequences of the heart on long-axis and short-axis, phase-contrast sequences for the evaluation of flows in specific planes, unenhanced CMR angiography, and contrast-enhanced sequences for the evaluation of fibrotic scars, the so-called late gadolinium enhancement (LGE), and myocardial perfusion studies. Cine sequences allow for the evaluation of morpho-functional parameters, such as ventricular volumes and ejection fraction, with a precision superior to that of echocardiography, especially with regard to the RV, and comparable to that of CCT. The main exclusive advantage of CMR, however, is the possibility to quantify flows: phase-contrast sequences are designed for the assessment of flow volumes and velocities through imaging planes, thus providing insight on valve function and possible stenosis or regurgitation. CMR angiography provides an accurate anatomic outline of the heart and vessels, whereas LGE and myocardial perfusion offer information concerning myocardial viability.
More recently, novel CMR sequences have been introduced to better assess the 3-dimensional morphology and the functionality of the heart and its vessels. Among those, free-breathing whole heart magnetic resonance angiography, which allows for excellent visualization of finer structures, such as coronary arteries, and 4-dimensional flow, which may add additional information regarding blood flow compared with phase-contrast sequences.
Nearly all of the recent implantable devices are CMR-safe or at least CMR-conditional; however, they often produce significant imaging artifacts. The typical contrast-enhanced CMR examination requires the intravenous administration of a gadolinium-based contrast agent; doses ranging from 0.1 to 0.2 mmol/kg are reported in the literature; however, considering the risk of potential accumulations of gadolinium in the brain, due to the frequency of repeated examinations in these patients, doses not exceeding 0.1 mmol/kg should be used.
Spectrum of repaired congenital heart disease
Tetralogy of Fallot
ToF is the most common cyanotic CHD in children, occurring in approximately 5 in 10,000 live births, and representing 5% to 7% of all CHD. ToF is caused by anterosuperior deviation of the conal septum, and this malalignment results in overriding of the interventricular septum by the aortic roof, obstruction of the pulmonary outflow tract, interventricular defect, and RV hypertrophy. Right-sided aortic arch, atrial septal defect (ASD), a secondary ventricular septal defect (VSD), or atrioventricular canal defects also can be associated with ToF. With regard to coronary anomalies, the most significant is the left anterior descending artery arising anteriorly off the right coronary artery. When present, it travels anterior to the right ventricular outflow tract (RVOT) and is at risk of injury during sternal entry, it is seen in 3% of ToF cases. As such, any 3-dimensional imaging performed in this patient population should mention the coronary anatomy. Progressive aortic root dilation also may occur.
The nature and timing of surgical repair has changed over the past few decades. In the current era, patients undergo primary repair typically by the first year of life or when they develop symptoms. A palliative approach may be considered when surgical repair is not immediately possible, with its aim being the establishment of a reliable source of pulmonary blood flow, thereby reducing the cyanosis. Palliative treatment consists of creation of shunts between systemic and pulmonary circulation, the most common of which are the Blalock-Taussig shunt, connecting the subclavian artery directly to a pulmonary artery, and especially in more recent years, the modified Blalock-Taussig shunt, which uses a Gore-Tex interposition graft. Pulmonary artery distortion is often seen in patients with prior palliative shunts and thus architecture of the branch pulmonary artery should be evaluated.
Surgical repair in patients with ToF involves closure of the VSD and relief of the RVOT obstruction ( Fig. 1 ). In prior decades, repair was done through a large RV incision and placement of a transannular patch. This routinely resulted in RV dysfunction and free pulmonic insufficiency. In the modern era, large transannular patches are avoided and a full degree of competency of the pulmonary valve is the aim.
General radiologists should be aware of the possible anatomic findings they might face while imaging a patient with ToF at any repair stage, namely before palliation, between palliation and surgery, or after surgery, and of the possible complications of every stage. When imaging a patient who underwent ToF palliation without complete repair, it is important to assess the status of the shunts and their patency, as the preservation of a constant source of blood to the pulmonary circulation is of utmost importance. In current era of adults with repaired ToF, the most common complication related to surgical ToF repair is severe pulmonary regurgitation. The primary purpose of advanced imaging focuses on this point, as timing of pulmonary valve replacement, as well as the nature of the surgical or percutaneous intervention, is dependent on 3-dimensional imaging.
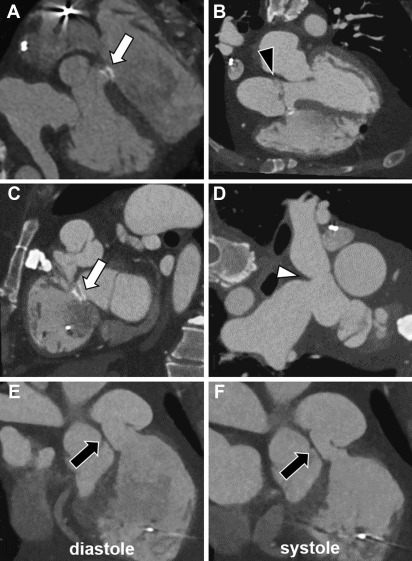
CMR represents the most common technique for the morpho-functional assessment of the RV in patients with repaired ToF through cine sequences. It is important to report RV volumes, ejection fraction, and degree of pulmonary regurgitation. Regurgitant fraction of greater than 48% is considered severe. MR angiography is also considered standard in the evaluation of repaired ToF. In asymptomatic patients, indications for pulmonary valve replacement (PVR) in the presence of moderate to severe pulmonary regurgitation often include RV end diastolic volume index of greater than 160 mL/m 2 or evidence of RV dysfunction ( Fig. 2 ). Contrast-enhanced CMR also allows the assessment of myocardial scar and diffuse fibrosis, where myocardial scar has been shown to relate to systolic dysfunction, and both scar and diffuse fibrosis are related to the insurgence of arrhythmias.
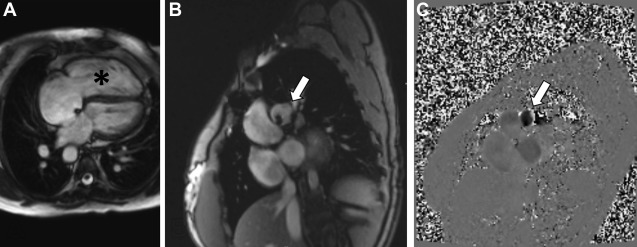
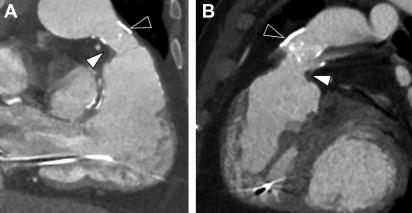
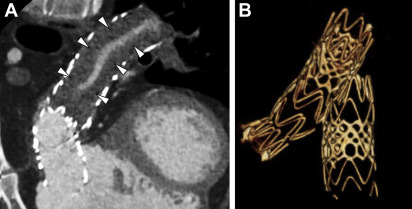
CCT also can provide accurate estimates of cardiac volumes and function. In patients with repaired ToF, it is well suited to evaluate for coronary artery anomalies or coronary disease. It is also commonly used in postoperative assessment of patients with ToF ( Fig. 3 ). Structurally, CCT well defines the anatomy of the RVOT, pulmonary valve annulus, and the branch pulmonary arteries ( Fig. 4 ). The severity and distribution of pulmonary artery calcifications and relationship between the RVOT and coronary arteries are well seen.
Transposition of the Great Arteries
Transposition of the great arteries (TGA) is a CHD in which the aorta arises from the RV and the pulmonary artery arises from the left ventricle (5% of all patients with CHD). Commonly associated defects include VSDs, left ventricular outflow tract obstruction, and coarctation of the aorta. In addition, the anatomy of the coronary arteries is variable.
The most common form of TGA is dextro-TGA (D-TGA), in which atrial-ventricular concordance is preserved, but the resultant ventriculo-arterial discordance results in cyanosis. In essence, there are 2 parallel circulations of blood flow with deoxygenated blood remaining in the systemic circuit, and oxygenated blood remaining in the pulmonary circuit.
Initial surgical palliations of D-TGA involved the creation of an atrial-level baffle in which blood from the systemic veins (inferior vena cava and superior vena cava) is rerouted to the posterior left ventricle and blood from the pulmonary veins is rerouted to the anterior RV. The RV remains the systemic ventricle and the left ventricle remains the subpulmonic ventricle. Two types of repairs are described, in the Mustard procedure, the atrial septum is excised, and pericardial tissue is used to create the atrial-level baffle. The Senning procedure is considered more complicated, but results in the same final solution. From an advanced imaging standpoint, they look nearly identical, and without operative history are difficult to distinguish.
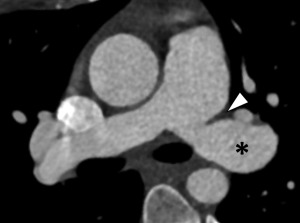
Current era surgical repair for D-TGA is arterial switch, in which the aorta and the main pulmonary artery are cut above their sinuses and transposed with the pulmonary artery placed anterior to the aorta ( Fig. 5 ). The coronary arteries are detached from the native aorta with some surrounding tissue and then sutured into place on the posterior neo-aortic root. The Rastelli procedure is a surgical intervention that palliates TGA along with VSD and left ventricular outflow tract obstruction when such concomitant issues are present ( Fig. 6 ).
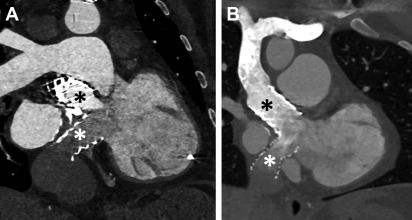
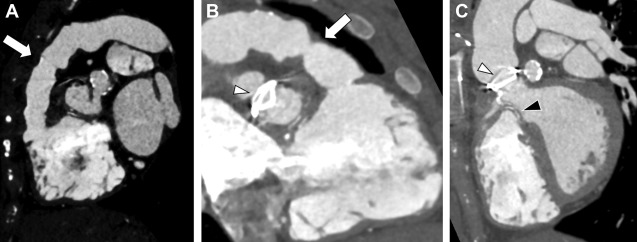
Complications of TGA repair are directly related to the type of surgical repair. In those with Mustard/Senning repairs, narrowing of the atrial baffles or baffle leaks may occur, although the most sinister finding is the development of systemic RV dysfunction. In patients with an arterial switch, coronary ostial stenosis related to the arterial anastomosis or supravalvular obstruction at the site of the aortic/pulmonic transection may occur. Systemic valvular disease, tricuspid valve regurgitation, is also common. Branch pulmonary stenosis also can be seen ( Fig. 7 ).
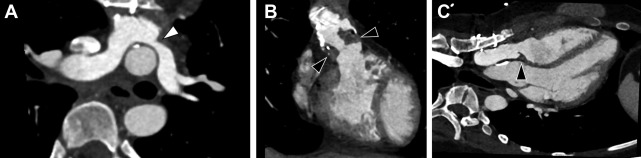
In patients with repaired TGA, CCT can provide accurate information with regard to cardiovascular anatomy and function, and it can help identify narrowing in baffles or baffle leaks, and calcifications ( Fig. 8 ). , Moreover, CCT can be used to assess coronary arteries and pulmonary arteries in view of a potential compression or stenosis due to surgical intervention ( Fig. 9 ). CCT is especially indicated in those patients who have implanted cardiac devices, as they may cause image artifacts, which account for up to 15% of individuals with repaired TGA, and in those in whom noncardiac thoracic pathology is likely to be a major issue.