J. Hodler, G. K. von Schulthess and Ch. L. Zollikofer (eds.)Musculoskeletal Diseases 2013–2016Diagnostic Imaging and Interventional Techniques10.1007/978-88-470-5292-5_21
© Springer-Verlag Italia 2013
Magnetic Resonance Imaging of Articular Cartilage
Michael P. Recht1
(1)
Department of Radiology, NYU Langone Medical Center, New York, NY, USA
Abstract
Magnetic resonance (MR) imaging has proven to be an accurate noninvasive method for the evaluation of articular cartilage [1–13], demonstrating morphologic changes as well as biochemical and structural changes. This chapter will concentrate on the morphologic imaging of articular cartilage and articular cartilage repair procedures using MR. Multiple MR pulse sequences have been used to evaluate the morphology of articular cartilage, but the most widely used clinically are proton density or T2-weighted fast spin-echo (FSE, TSE) sequences with or without fat suppression.
Magnetic resonance (MR) imaging has proven to be an accurate noninvasive method for the evaluation of articular cartilage [1–13], demonstrating morphologic changes as well as biochemical and structural changes. This chapter will concentrate on the morphologic imaging of articular cartilage and articular cartilage repair procedures using MR. Multiple MR pulse sequences have been used to evaluate the morphology of articular cartilage, but the most widely used clinically are proton density or T2- weighted fast spin-echo (FSE, TSE) sequences with or without fat suppression.
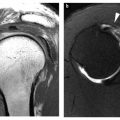
Fast spin-echo sequences employ multiple refocusing pulses that allow high-resolution images to be acquired in a relatively short time period. In addition, the multiple refocusing pulses produce a magnetization transfer effect within articular cartilage, which decreases its signal intensity. There is no corresponding effect within joint fluid, and therefore the magnetization transfer effect increases the contrast between articular cartilage and joint fluid, and improves the conspicuity of chondral defects. Studies have shown both proton density and T2-weighted fast spin-echo images with and without fat suppression to be accurate in the detection of chondral abnormalities. On fast spin echo images, cartilage has intermediate signal intensity tissue and is outlined by high signal intensity joint fluid providing excellent depiction of surface morphology as well as intrinsic signal changes potentially reflective of intrasubstance pathology. Chondral lesions appear as focal areas of increased signal intensity compared with normal surrounding cartilage. Although images without fat suppression have been shown to be accurate, the addition of fat suppression improves the ability to detect underlying subchondral bone marrow signal changes, which have been shown to be an important indicator of overlying articular cartilage defects. Studies have demonstrated a sensitivity of 86–94%, specificity of 94–99%, and accuracy of 81–98% for the detection of cartilage abnormalities on fast spin-echo techniques.
Over the past several years, three-dimensional (3D) fast spin-echo sequences have been developed, and these offer the promise of isotropic resolution and multiplanar reconstructions without loss of resolution while maintaining the excellent contrast of two-dimensional (2D) fast spin-echo sequences with reasonable times of acquisition (6–8 min) [14–16]. Initial studies have demonstrated equivalent diagnostic performance with 2D fast spin-echo sequences. 3T MR imaging and multichannel coils have also been utilized in cartilage imaging to take advantage of the potential advantages of imaging with relative increased image signal-to-noise or higher spatial resolution at similar imaging acquisition times, compared with 1T or 1.5T imaging (albeit with somewhat increased sensitivity to postoperative metal-related artifacts) [17, 18].
Clinical MR Imaging of Articular Cartilage
Degenerative Cartilage Disease
Degenerative cartilage disease or osteoarthritis is the most common form of arthritis, with symptomatic disease affecting 6–10% of all adults greater than 30 years of age, and illustrating a steep increase in prevalence with increasing age [19]. Early changes of osteoarthritis may be detected as areas of surface irregularity and fibrillation, as well as small focal defects presenting as focal areas of increased signal intensity on proton density or T2- weighted fast spin-echo images. More advanced changes of osteoarthritis include large areas of chondral thinning, often on apposing surfaces of the joint, and multiple focal defects of varying size. The focal defects tend to have obtuse margins as opposed to the sharply angled margins of traumatic chondral lesions. There is frequently increased signal abnormality subjacent to the focal defects within the subchondral bone marrow representing cysts and/or subchondral bone marrow edema like changes, and/or low signal representing subchondral fibrosis or trabecular sclerosis. The presence of additional secondary features of degenerative disease, such as osteophytes, and synovitis are also frequently identified on MR imaging.
Traumatic Cartilage Injury
Traumatic articular cartilage injuries are well recognized sequella of acute or repetitive impact or twisting injuries to a joint and are a significant source of patient morbidity. Surgical studies have highlighted that cartilage lesions are common, with an incidence ranging from 63% to 66% in patients undergoing arthroscopic surgery [20, 21]. Traumatic chondral lesions typically present as solitary lesions with acutely angled margins. The lesions usually result from shearing, rotational or tangential impaction forces, and can occur at the surface or in the deeper layers of articular cartilage. Traumatic lesions are often full thickness or high-grade partial thickness tears, and can frequently involve the underlying subchondral bone with either subchondral bone marrow edema like changes, contusions or frank fractures. Chondral fractures may remain in situ or become displaced, and present as intraarticular chondral or osteochondral bodies, which may cause locking of the knee and masquerade as a bucket handle meniscal tear.
Imaging of Cartilage Repair Procedures
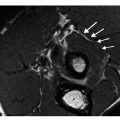
Increased awareness of the prevalence and significance of articular cartilage lesions, coupled with the limited natural capacity of cartilage for effective intrinsic repair, has contributed to growing interest in surgical techniques for the treatment of articular cartilage lesions. There are several techniques used to treat cartilage lesions; these techniques can be grouped into three main categories: local stimulation, autologous transplantation of cartilage, and allograft transplantation of osteochondral grafts. The most commonly used techniques are those of local stimulation, but these typically lead to formation of fibrous repair tissue rather than hyaline cartilage. Therefore, there has been great interest and increased use of autologous transplantation techniques, including autologous osteochondral transplantation and autologous chondrocyte implantation, because of their potential for providing hyaline or „hyaline-like” repair tissue and the promise of better functional results. In addition to a routine assessment of joint anatomy, a complete MR imaging evaluation of cartilage repair procedures should include specific assessment of the following. (a) Repair tissue: defect fill, surface morphology, MR signal characteristics. (b) Adjacent cartilage and bone: repair tissue integration to native cartilage and subchondral bone, MR signal characteristics subchondral bone. (c) The articulation: joint effusion, synovitis, adhesions, loose bodies.
Local Stimulation for Cartilage Repair
The most commonly used surgical procedure for local stimulation is microfracture, which relies on bleeding from the penetration of the subchondral bone to form a fibrin clot containing pleuripotent stem cells. This clot differentiates and remodels leading to the formation of fibrocartilaginous repair tissue. With microfracture, a pick instrument is used to penetrate the subchondral bone multiple times about 4 mm in depth and approximately 3– 4 mm apart from each other [22].
In the first few months following the microfracture procedure, repair tissue forms that is typically thinner than the adjacent native articular cartilage and is of intermediate signal intensity [23]. Over time, the amount of repair tissue increases with the optimal result being 100% defect fill with a congruent articular surface and repair tissue of similar signal intensity compared with native articular cartilage. It is common to see edema-like signal change within the subchondral bone following the procedure, though this usually resolves over several months [23]. Failure of the microfracture procedure is demonstrated as poor fill of repair tissue, often with fissuring and chondral flaps.
Autologous Osteochondral Transplantation
Autologous osteochondral transplantation involves the harvesting and reimplantation of osteochondral plugs to repair chondral defects. The osteochondral plugs are harvested from a relative non-weight-bearing area of the joint, typically the intercondylar notch region or the borders of the femoral trochlea. The chondral defect being repaired is debrided and the osteochondral plugs are transplanted into the defect site. The orientation, position and number of osteochondral plugs are important determinants of the outcome of the procedure. The goal of the procedure is to create a congruent cartilage surface and therefore the plugs need to be placed perpendicular to the surface. Because the plugs typically come from a region of the knee joint where the articular cartilage is thinner than the recipient site, in order to have a flush articular surface there is often an incongruent bone-bone interface. Histologic evaluation of autologous osteochondral transplants has shown viable graft hyaline cartilage with the interstices between graft plugs filled with fibrocartilage-like repair tissue [24, 25]. Therefore, it is desirable to fill as much of the defect as possible with the plugs. However, the number of plugs that can be used is limited by the availability of donor sites and the need to limit morbidity at the donor sites.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree