Fig. 1
Grade 2 muscle strain. 26-year-old man injured playing soccer 4 days prior to magnetic resonance imaging. Axial PD-weighted fat-suppressed image of the left thigh shows peritendinous and epimyseal fluid involving the hamstring musculature. Note the relative preservation of the muscle architecture
Grade 3 strains are the least common type of injury. These complete ruptures are usually characterized by retraction and waviness of torn fibers, as well as substantial hemorrhage in the acute setting. With MRI, the injury grade (as well as the length and crosssectional area of edema signal) often correlate with rehabilitation time.
The age of a patient influences the location of injury along the biomechanical chain formed by the linkage of muscle to tendon and tendon to bone:
In older adults (or others with tendinopathy), the „weak link” is the degenerated tendon. Tendinopathy becomes more prevalent with advancing age, and so do tendon tears (e.g., rotator cuff tears through pre-existing tendinopathy).
In adolescents, the weakest link in the chain tends to be the physis, and therefore apophyseal avulsions are common.
In young adults (who have healthy tendons and closed apophyses), strains characteristically target the junction of muscle and tendon tissue (myotendinous junction), because strong muscles generate particularly high „strain” forces where these tissues with different physical properties interface. There are three locations for these interfaces: the classic myotendinous junction (where tendons emerge from muscle proximally and distally), the intramuscular myotendinous junction (along tendon slips that run within the muscle), and the myofascial junction (at the periphery of the muscle).
In one study of young athletes, acute hamstring injuries occurred at the myotendinous junction in diverse locations: the proximal myotendinous junction (33%), the intramuscular myotendinous junction (53%), and the distal myo — tendinous junction (13%) [8]. Note that this is in contradistinction to older adults; their hamstring injuries occur most commonly at the proximal tendon attachments, owing to underlying tendinopathy. Although the vast majority of muscle strains resolve with only nonoperative management, a common operative indication is an avulsion and a tear in the tendon, which are amenable to treatment with suture anchors and direct suture repair, respectively.
The most commonly strained muscles in the extremities include the rectus femoris, hamstrings, adductors and gastrocnemius muscles [9].
Rectus Femoris Strain
The proximal rectus femoris has two heads. The direct (or „straight”) head arises from the anterior-inferior iliac spine, and contributes to the anterior fascia of the muscle. The indirect (or „reflected”) head arises from the superior acetabular ridge and hip capsule, contributing to a long musculotendinous junction within the muscle [10]. Rectus femoris strains targeting this deep intramuscular musculotendinous junction (sometimes referred to as the central tendon or aponeurosis) can result in a „bull’s-eye sign” on MRI, and have a longer rehabilitation time than strains located at the muscle periphery [11].
Hamstring Strain
The hamstrings are composed of the semimembranosus, semitendinosus and biceps femoris [12–14]. The semimembranosus origin is the superolateral facet of the ischial tuberosity. The semitendinosus and biceps femoris long head originate from a conjoint tendon at the inferomedial facet of the ischial tuberosity. High-grade or complete tendon rupture (or bony avulsion) at the proximal hamstring complex is considered an indication for surgical repair in active young adults, with better results in the acute setting [13].
The hamstrings are the most commonly injured muscles in sprinting and jumping athletes. Of the three hamstring components, the biceps femoris long head is the most commonly injured in the classic abrupt-onset hamstring strain. Less commonly, in some sports (e.g., dance), the onset of a stretching injury may occur more slowly, target the proximal semimembranosus tendon, and take much longer to heal [15]. Rehabilitation time may be suggested by the extent of hamstring injury seen by MRI. Convalescence periods reportedly vary widely from 2 weeks to 1.5 years before patients can return to vigorous activities. Recurrent injuries are common, occurring in one-quarter of athletes. Even minor hamstring injuries may double the risk of a more severe injury within 2 months.
Calf Muscle Strain
Several muscles and tendons at the posterior aspect of the knee and calf may be subjected to strain injuries, including the gastrocnemius, soleus, plantaris, and popliteus muscles. „Tennis leg” may be defined clinically as the sudden onset of sharp pain in the mid-calf while participating in athletics (e.g., racket sports, running), typically in middle-aged persons [16]. Most commonly, these injuries are partial tears involving the (fast-twitch) medial head of the gastrocnemius muscle. Isolated strain of the (slow-twitch) soleus muscle is less common, usually occurring with endurance activities [17]. Treatment is almost always conservative, typically with relief of pain within 2 weeks and return to sports after at least 3 weeks. Clinical differential diagnosis may include a ruptured Baker’s cyst, overuse „tendinitis”, stress fracture, chronic exertional compartment syndrome, fascial herniation, venous thrombosis, nerve entrapment and popliteal artery entrapment syndrome.
Avulsion
Given that the physis is a weak link in the biomechanical chain for skeletally immature patients, we commonly see apophyseal avulsion fractures in adolescent athletes. The pelvis, with its many apophyses, is a common site for such avulsions. In one study of 203 adolescent athletes with acute pelvic avulsion fractures, the most common sites were: (1) the ischial tuberosity (origin of the hamstrings), (2) the anterior-inferior iliac spine (origin of the rectus femoris), and (3) the anterior-superior iliac spine (origin of the sartorius) [18]. Such avulsions generally are treated conservatively and have a good prognosis, although nonunions can occur. The imaging appearance of osseous avulsion injuries may be mistaken for a neoplastic or infectious process, especially in the nonacute setting when no history of trauma is provided. Knowledge of the major tendinous attachments to bone is indispensible in arriving at a correct diagnosis — and avoiding misdiagnosis of an osteochondroma or osteosarcoma.
Contusion
Unlike muscle strains caused by indirect (noncontact) injury, contusions are caused by direct concussive trauma, usually by a blunt object. The resultant interstitial edema and hemorrhage correspond to the site of impact (rather than being localized to the myotendinous junction). Hemorrhage can be seen in the muscle, intermuscular fat planes or the subcutaneous tissues. Soft tissue hemorrhage can collect focally, resulting in a hematoma.
Sequelae of Musculotendinous Injury
Several sequelae of musculotendinous injury may be observed, including hematoma, heterotopic ossification, fibrosis and atrophy. These sequelae are not specific to the post-traumatic setting, but rather reflect the limited pathological responses that muscle has when confronted with a wide array of insults.
Hematoma
Intramuscular hematomas evaluated between 5 days and 5 months after injury commonly display characteristics of methemoglobin, with increased signal intensity on both T1- and T2-weighted images. Most intramuscular hematomas tend to diminish in size over a period of 6– 8 weeks. Differentiation between a simple hematoma and a hemorrhagic neoplasm may be difficult in some patients both clinically and with imaging. Administration of contrast material aids in excluding a neoplasm when the lesion in question shows no enhancement. Conversely, the presence of an enhancing nodule in a muscle lesion may suggest the diagnosis of a neoplasm rather than a hematoma (Fig. 2). When the diagnosis of a probably benign hematoma is in doubt, clinical correlation and a follow- up MRI examination are indicated to confirm lesion stability or resolution. In the chronic setting, serous-appearing fluid may linger within a connective tissue sheath, creating an intramuscular pseudocyst or „seroma”. Heterotopic ossification also may occur.
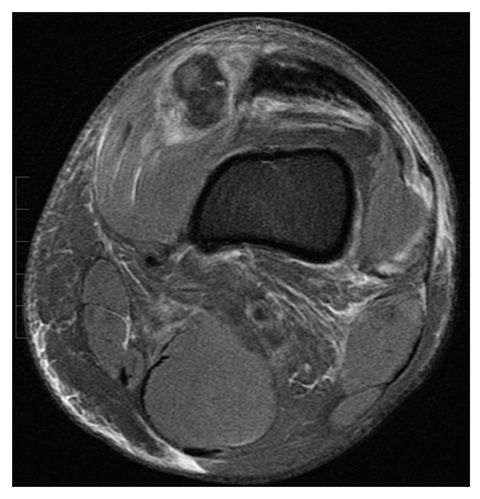

Fig. 2
Muscle hematoma. Axial proton-density-weighted fat-suppressed image of the left thigh obtained 1 day after a direct blow to the medial knee shows an acute hematoma in the vastus medialis muscle, with surrounding edema. Note signal heterogeneity, with some regions of higher signal representing early methemoglobin formation
Heterotopic Ossification
The most common type of heterotopic ossification occurs in muscle, and commonly is referred to as myositis ossificans. Risk factors for heterotopic ossification include traumatic insults (e.g., contusion, surgery, burns), neurological insults (e.g., paraplegia, traumatic brain injury, stroke), or bleeding dyscrasias (e.g., hemophilia). In the clinical and radiological arenas, three typical phases of evolution occur: (1) an acute or pseudo-inflammatory phase; (2) a subacute or pseudotumoral phase; and (3) a chronic, self-limited phase that may (or may not) undergo spontaneous healing. In the acute and subacute stages of myositis ossificans, imaging examinations have a notoriously nonspecific appearance. CT is the single best imaging examination for showing the characteristic ossific matrix. In the final stage, the imaging findings that permit confident differentiation of myositis ossificans from neoplasm are: (1) the ossific mass is well-defined, sharply marginated, and appears more mature peripherally than centrally with architecture that approximates native bone (an area of cancellous bone centrally surrounded by compact bone peripherally); (2) the lesion generally decreases in size with the passage of time; and (3) there is no destruction in the underlying bone.
Imaging findings evolve over time, and are typically nonspecific in the acute and subacute stages. The involved muscle is enlarged and underlying periostitis is common. With MRI, the muscle exhibits nonspecific intermediate T1 and high T2 signal intensity. As the lesion matures, T2 hyperintensity and contrast enhancement progressively decrease. The signal intensity of the lesion may remain inhomogeneous, although areas of signal intensity equivalent to marrow fat and cortical bone increase [19]. Mature myositis ossificans is easiest to recognize when there is a low signal rim and fat signal centrally, although persistent granulation-type tissue may make the diagnosis difficult by MRI.
Myositis ossificans has been designated a „don’t touch” lesion that should not be biopsied injudiciously. Treatment for myositis ossificans may include physical therapy, nonsteroidal antiinflammatory agents, bisphosphonates, low-dose irradiation therapy and, in uncommon cases, surgical resection for a bulky area of ossification that causes nerve entrapment or limits range of motion. Surgical resection of myositis ossificans traditionally is performed after the mass „matures” in the hopes of minimizing the risk of recurrence.
Fibrosis
Fibrosis is characteristically displayed as low signal intensity tissue in muscle on T2-weighted images after a nonacute insult. Recognized sites of muscle fibrosis include the deltoid, gluteus maximus and the vastus lateralis. Evaluation of the clinical impact and treatment of fibrosis is an active area of research.
Atrophy
Muscle atrophy may occur after certain musculotendinous injuries, disuse or other insults. The cardinal feature of muscle atrophy is decreased muscle volume, which is often accompanied by fatty infiltration. The most frequent site of muscle atrophy is in the shoulder girdle after a rotator cuff tear. After a supraspinatus tendon tear, adjacent muscle atrophy is recognized as a negative prognostic factor for patients undergoing cuff repair. Atrophy of other shoulder girdle muscles also can occur, even when the rotator cuff tendons are intact. Muscle atrophy begins to develop within 10 days after immobilization. After bed rest for 20 days, the muscle cross-sectional area decreases approximately 10% in healthy men [20]. Muscle atrophy may be partially irreversible by 4 months.
Muscle Ischemia and Myonecrosis
Compartment Syndrome
Compartment syndrome is defined as elevated pressure in a relatively noncompliant anatomic space that is associated with ischemia, and may result in neuromuscular injury, including myonecrosis and rhabdomyolysis [21]. Risk factors for compartment syndrome include a history of trauma, external compression, systemic hypotension, increased intracompartmental volume (e.g., hemorrhage, edema, poor venous return, muscle hypertrophy), and loss of compartment elasticity (e.g., fibrotic or constricted fascia). Compartment syndrome can occur at virtually any location, most commonly in the leg and less commonly in other sites such as the thigh, forearm and paraspinal musculature (Fig. 3).
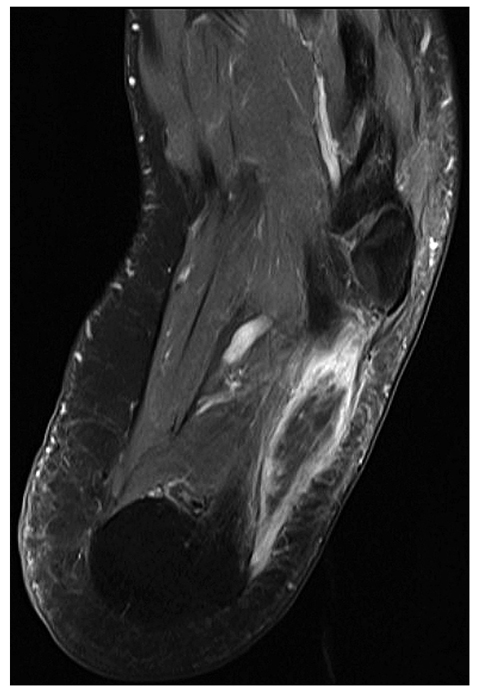

Fig. 3
Compartment syndrome. 40-year-old man with foot pain following prolonged surgical procedure of pelvis done with patient in lithotomy position with foot blocks. Axial T1-weighted fat-suppressed magnetic resonance image following intravenous contrast enhancement shows intense peripheral enhancement limited to the periphery of the abductor digiti quinti muscle. Similar changes were present in the contralateral foot (not illustrated) related to compression-induced compartment syndrome
Patients initially complain of painful aching, tightness, or pressure that worsens with palpation and passive stretching of the affected muscles.
Compartment syndrome is generally classified as acute or chronic:
Acute compartment syndrome is a surgical emergency treated by fasciotomy, which decompresses the hypertensive muscle and improves perfusion. Although most cases of acute compartment syndrome are associated with fractures, the second most common cause is injury to soft tissues (e.g., contusion) without fracture. The definitive diagnosis is made with direct percutaneous compartment pressure measurements that may be aided by near infrared spectroscopy.
Chronic compartment syndrome may occur as a result of exertional causes (e.g., exercise, occupational overuse) or, less commonly, „non-exertional” causes (e.g., a mass lesion, infection). The most common type of chronic exertional compartment syndrome, for example, occurs in the legs of running athletes. The thigh, forearm and foot are the next most common sites in athletes, depending on the muscles used in their chosen sport.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree