Blunt trauma accounts for more than 95% of traumatic renal injury and results from shear forces from rapid acceleration or deceleration and/or collision against the spine or ribs. The use of multiphasic contrast-enhanced computed tomography (CT) has proven pivotal in the evaluation and management of traumatic kidney injury, and CT imaging features provide the basis for nonsurgical staging. This article describes the epidemiology and mechanisms of blunt and penetrating traumatic renal injury and reviews the range of findings from various imaging modalities, with a particular emphasis on contrast-enhanced CT.
Key points
- •
The kidneys are the most commonly injured organ in the genitourinary system, with blunt injury accounting for the vast majority of these injuries.
- •
Multiphasic contrast-enhanced computed tomography (CT) is the imaging modality of choice for the detection of renal trauma. Both arterial and portal venous phase acquisitions are recommended to optimize sensitivity for the range of injury. The use of other imaging modalities, including ultrasound, dual-energy CT, MR imaging and conventional angiography, may be indicated in special situations but are much less common in practice.
- •
Excretory phase CT is important for the detection of collecting system injury and is selectively used based on findings from the arterial- and portal venous phase images.
- •
The American Association for the Surgery of Trauma (AAST) Organ Injury Scale (OIS), the most commonly used grading system for renal trauma, was updated in 2018 and incorporates imaging findings from contrast-enhanced CT, including parenchymal, collecting system, and vascular injury.
- •
The primary goal in the management of acute renal trauma is to optimize patient survival and preserve renal function. Imaging has played a pivotal role in a paradigm shift away from invasive surgical diagnosis and treatment toward nonoperative management.
Introduction
The kidneys are the most commonly injured organ in the genitourinary system, although renal injuries occur in only 1.2% to 3.25% of all trauma patients. , Renal trauma occurs at a mean patient age of 20 to 30 years and affects men at 3 times the rate of women. , The vast majority (71%–95%) of renal injuries occur secondary to a blunt mechanism, such as motor vehicle crashes and falls, and a minority are due to penetrating trauma, most commonly gunshot and stab wounds. , Due to their retroperitoneal location, bounded anteriorly by peritoneal organs and posteriorly by paraspinal musculature, thoracolumbar vertebrae, and ribs, the kidneys are relatively resistant to injury. Injury in blunt trauma likely results from shear forces from differential deceleration of vital structures, particularly around the relatively fixed renal hilum, or from crush injury against the spine and ribs. Most renal injuries are minor and clinically insignificant. More significant renal trauma usually occurs concomitantly with injuries to other abdominal organs.
In recent decades, there has been a steady and continuing paradigm shift from surgical intervention to nonoperative management for renal trauma. This trend has been facilitated by the widespread availability of computed tomography (CT) for the evaluation of trauma patients, improvements in conservative management approaches, and evidence that conservative management is safe and effective. The development of a validated renal injury scoring system based on surgical findings, the American Association for the Surgery of Trauma Organ Injury Scale (AAST OIS), has helped to standardize research and clinical treatment of renal injuries. As contrast-enhanced CT (CECT) findings have demonstrated a relatively strong correlation with intraoperative findings, , CECT has been largely relied on as a nonoperative proxy for estimating OIS grade and is now the modality of choice for the initial evaluation of clinically stable patients with suspected renal trauma.
Multiphasic contrast-enhanced computed tomography in renal trauma
The main goals of imaging in the setting of suspected renal trauma are to accurately stage and differentiate injuries requiring early operative versus conservative management, distinguish traumatic from preexisting renal pathology, assess the integrity and function of the contralateral kidney, and identify other associated abdominal organ injuries. Approximately 95% of patients with renal injury present with at least microscopic hematuria (≥5 red blood cells per high-powered field). It is important to note, however, that patients with disruption of the renal vascular pedicle or the ureteropelvic region may not present with hematuria. Accordingly, the degree of hematuria does not always correlate with the severity of renal injury. Generally accepted indications for imaging to evaluate for blunt renal injury include macroscopic hematuria, microscopic hematuria with either shock or coexisting abdominal organ injuries, and severe mechanism of injury with documented factors associated with renal trauma, such as posterior flank hematoma and rib or spinal fractures detected on radiography. In penetrating injury with a trajectory likely to involve the kidneys, CECT is indicated in hemodynamically stable patients regardless of the presence of hematuria.
CECT is readily available in essentially all institutions providing emergency services and can quickly, safely, and accurately depict renal and other injuries in the abdomen and pelvis. Radiology departments in trauma centers are increasingly adopting the use of arterial and portal venous phases in the evaluation of abdominal trauma, including renal trauma.
Dual-phase imaging can be achieved by either 2 separate CT acquisitions of the initial intravenous contrast bolus (conventional technique) or by a split-bolus technique that requires 2 separate intravenous contrast administrations and a single CT acquisition. which then contains simultaneous arterial and portal venous phase imaging information. Using a fixed-time delay protocol with the conventional technique, the arterial phase is acquired 15 to 20 seconds after the administration of intravenous contrast. This phase provides excellent delineation of arterial injury and, along with the portal venous phase acquisition, can help to differentiate active bleeding from contained vascular injury (pseudoaneurysm and arteriovenous fistulas [AVFs]). Renal parenchymal injury, however, is poorly evaluated on the arterial phase as it typically corresponds to a corticomedullary enhancement pattern of renal parenchyma. The portal venous phase acquisition (with a 70- to 80-second delay from the time of the contrast bolus administration), which corresponds to the nephrogenic or late corticomedullary enhancement pattern in most instances, is needed to optimize the detection of the range of renal parenchymal injuries, which are hypoattenuating on a background of the uniformly enhancing renal soft tissue.
The detection of renal collecting system disruption requires the addition of an excretory phase, which occurs between 5 and 10 minutes following the administration of intravenous contrast. The decision to proceed with an excretory phase is made on a case-by-case basis after a provisional review of the arterial and portal venous data set, and optimally while the patient is still in the scanner. Deep lacerations that appear to extend to the renal calyces or renal pelvis, or the presence of a significant volume of perinephric or periureteral simple or nonsimple fluid, are imaging findings that justify rescanning the patient in the excretory phase to assess for urinary extravasation.
Grading traumatic renal injuries
The most widely used classification system for renal trauma is the AAST OIS, first devised in 1989. This scale assigns a numerical score from 1 to 5, from least to most severe injury. Research has demonstrated that AAST renal grade correlates well with patient mortality and the need for nephrectomy. , Because the original version was published before the widespread use of CECT in the evaluation of trauma, scoring was based solely on operative findings. Since the first iteration, however, CECT has largely supplanted surgical exploration as the diagnostic standard for renal and other abdominal organ trauma. Furthermore, CECT is able to detect clinically relevant injuries that were not included in the original OIS. These include pseudoaneurysms, AVFs, active bleeding, and collecting system disruption, for which advances in minimally invasive management have been developed, particularly image-guided endovascular embolization. Recognizing the impact of these injuries on patient outcomes and the reliability of CECT for their detection, the AAST OIS Patient Assessment Committee convened in 2015 to revise the OIS, publishing the first revised version in 2018. The revised OIS encourages the use of CECT for grading renal trauma and sets out specific CECT-based criteria for this purpose. As in the original version, the new OIS advises to upgrade coexisting grade 1 or 2 injuries by 1 point (up to grade 3), when assigning the final organ injury grade. The 2018 AAST OIS for the kidney is summarized in Table 1 and Fig. 1 .
| Grade | Type | CECT Imaging Features |
|---|---|---|
| 1 | Contusion Hematoma | Isolated parenchymal contusion Subcapsular hematoma |
| 2 | Laceration Hematoma | Laceration <1 cm without extension to collecting system Perinephric hematoma contained by Gerota fascia |
| 3 | Laceration Vascular | Laceration >1 cm without extension to collecting system Any grade 1–3 with PSA/AVF or active bleeding contained by Gerota fascia |
| 4 | Laceration Vascular | Laceration with extension to collecting system Renal pelvis laceration or complete ureteropelvic laceration Segmental renal artery or vein intimal injury/thrombosis Active bleeding beyond Gerota fascia Segmental or complete renal infarction from vessel thrombosis without active bleeding |
| 5 | Laceration Vascular | Shattered kidney Main renal artery or vein laceration or avulsion from renal hilum Complete organ devascularization with active bleeding |

Contrast-Enhanced Computed Tomography
The full range of renal parenchymal, vascular, and urinary collecting system injury have characteristic features on CECT, and are described as follows:
- •
Contusion : Renal contusions are the most minor form of parenchymal injury and represent focal areas of bruising. On CECT in the portal venous (or nephrogenic) phase, contusions appear as focal, indistinctly marginated, subtly hypodense regions, reflecting areas of hemorrhage intermixed with uninjured tissue ( Fig. 2 ). Clotted blood in a contusion may appear isodense to normally enhancing parenchyma and, therefore, may be missed. A contusion is often inapparent on the arterial phase due to the suboptimal enhancement of background renal parenchyma, again underscoring the importance of a portal venous phase for the detection of tissue injury. On the excretory phase, a focal “delayed nephrogram” of retained contrast may be demonstrated, indicating impaired localized parenchymal function. A contusion reflects an AAST grade 1 injury.

Fig. 2
Gunshot wound to the right flank depicting renal contusion. Axial ( A ) and sagittal ( B ) CECT demonstrating a contusion ( arrow ) in the posterolateral right kidney as a focal relatively hypoattenuating region with indistinct margin, but with density greater than that of bile in the gallbladder in ( A ) and simple fluid in a hepatic cyst in ( B ). Embedded metallic fragments and soft tissue gas are present in the right posterior chest wall superficial to the contusion in ( A ). Bullet track through the posterior aspect of the right hepatic lobe in ( B ) is present adjacent to the renal contusion. A renal contusion is classified as an AAST grade 1 injury.
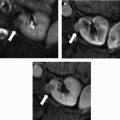
- •
Laceration : A laceration appears as an irregular or branching linear hypoattenuation on the portal venous phase of CECT ( Fig. 3 ). Lacerations are classified by length and whether they extend to the renal collecting system. Although collecting system involvement can often be inferred when the deepest portion of a laceration extends to a calyx or the renal pelvis (and/or when there is an associated adjacent fluid collection suspicious for extravasated urine), an excretory phase is required for confirmation. Lacerations can be a feature of AAST grades 2 to 5 .

Fig. 3
Axial CECT illustrating a laceration extending from the posterior aspect of the kidney to the periphery of a renal pyramid ( arrow ). Because this laceration measures less than 1 cm in length and clearly does not extend to a calyx or pelvis, this injury would be classified as AAST grade 2.
- •
Subcapsular hematoma : A subcapsular hematoma is contained by the renal capsule peripherally and exerts mass effect on the underlying renal parenchyma. This results in a characteristic eccentric, crescentic, or biconvex appearance of the hematoma on CT ( Fig. 4 ). The hemorrhage within a subcapsular hematoma may be unclotted (30–50 Hounsfield units [HU]) or clotted (50–70 HU). A subcapsular hematoma represents an AAST grade 2 injury .

Fig. 4
Axial CECT demonstrating a subcapsular hematoma along the lateral aspect of the left kidney ( arrow ). The renal contour is maintained and there is no perinephric hemorrhage, consistent with a subcapsular hematoma with an intact capsule. This injury is consistent with an AAST grade 1 injury.
- •
Perirenal hematoma : A perirenal, or perinephric, hematoma represents accumulated blood products around the kidney, and is further classified as being contained by Gerota (perirenal or perinephric) fascia or extending beyond Gerota fascia within the retroperitoneum or into the peritoneal cavity. A perirenal hematoma is characterized by a poorly marginated, hyperattenuating collection superficial to renal capsule. This finding is rarely present in isolation and more commonly occurs in association with parenchymal lacerations and/or vascular injury ( Fig. 5 ). A perirenal hematoma can be a feature of grades 2 to 5.

Fig. 5
Axial CECT depicting a perirenal hematoma ( arrow ). The edges of a perirenal hematoma are ill-defined and do not exhibit significant mass effect on the kidney, in contrast to a subcapsular hematoma. Perirenal hematomas are further classified as either contained by Gerota fascia (present case) or extending beyond Gerota fascia. Most perirenal hematomas occur along with other renal injuries, as in this instance, in which a laceration along the posteromedial aspect of the kidney is present. Ordinarily, an isolated perirenal hematoma would be classified as AAST grade 2; however, because in this case it is associated with a low-grade laceration (grade 2), the injury complex would advance 1 grade from grade 2 to 3.
- •
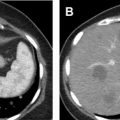
Renal collecting system injury : Recognizing the presence of renal collecting system injury is important because it reflects high-grade injury that may require intervention with urinary diversion. Lacerations that appear to extend to the collecting system on nephrographic phase of imaging raise the suspicion of collecting system injury and should be further evaluated with excretory phase imaging. The presence of a significant amount of perirenal hemorrhage or simple fluid is an additional imaging feature that is concerning for collecting system injury. On excretory phase, collecting system injury is confirmed by the presence of excreted contrast beyond the collecting system ( Fig. 6 ). In complete ureteropelvic disruption, excreted contrast will accumulate in the region of wall injury but will not be present in the ureter distal to the injury. In partial pelvic or ureteropelvic tears, contrast may opacify the entire ureter because a portion remains intact. Collecting system injury may be a feature of AAST grade 4 or 5 injuries.

Fig. 6
Urinary extravasation from renal collecting system injury. Axial CECT in the portal venous/nephrographic phase ( A ) and excretory phase ( B ). In ( A ), extensive lacerations traverse the inferior pole of the left kidney. A perinephric hematoma is present along the posterior margin of the kidney. In ( B ), excreted contrast leaks out of the collecting system and accumulates along the inferomedial aspect of the kidney ( arrow ). Urinary collecting system involvement is a feature of only AAST grade 4 or 5 injury.
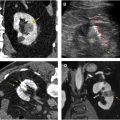
- •
Renal infarction : Renal infarction is characterized as either segmental or complete. A segmental infarct reflects injury to a segmental renal artery and results in a characteristic wedge-shaped region of parenchymal hypoattenuation with the apex directed toward the hilum ( Fig. 7 ). A segmental infarct will remain hypoattenuating on all phases of imaging, which aids in distinguishing it from a contusion. Complete renal infarction occurs as a result of severe vascular injury (main renal artery or vein avulsion or thrombosis) and is characterized by the absence of enhancement of the affected kidney on all phases (see Fig. 7 ). Complete infarction, particularly when associated with active bleeding from the main renal vessels, may necessitate emergent nephrectomy. A segmental renal infarct is currently classified as AAST grade 4 ; however, there is some controversy over assigning it such a high grade because these types of injury generally heal with conservative management and without clinically significant sequela. Complete renal infarction without active bleeding reflects an AAST grade 4 injury, whereas with active bleeding, it is classified as grade 5.