(1)
Princess Elizabeth Hospital Le Vauquiedor St. Martin’s Guernsey, Channel Islands, UK
(2)
Brighton and Sussex Medical School, Brighton, England UK
Abstract
Benign breast lesions can present symptomatically or mammographically detected through screening programmes. Risk assessment is important for both the clinician and the patient to assist in making decisions regarding screening, prophylactic treatment and surveillance. Because of widespread use of mammography, clinically silent benign lesions are now easily detected, leading to pathological assessment to exclude the presence of malignancy. Fine needle aspiration cytology, needle core biopsies and excision biopsies are the methods used to confirm benignity and allay patient anxiety. When a woman has had a diagnosis of benign breast disease, more often than not she wishes to know what is the likelihood of the benign lesion predisposing to cancer at a later stage. With some conditions, such as fat necrosis, duct ectasia or hyalinised fibroadenoma, the clinician can reassure the patient confidently that there is no risk of developing cancer. However, some benign proliferative lesions confer an increased risk of subsequent malignancy, although in most cases the risk is very small. These conditions require careful explanation to the patient, not to alarm them but to encourage compliance with follow-up. The advantage of assessing the possible risk of progressing to malignancy in benign disease is that regular clinical and radiological follow-up can detect malignancy at an early, potentially curable stage.
Learning Points
Accurate assessment of risk of breast cancer due to benign breast lesions is affected by previous use of different terminology.
Accurate risk assessment is essential in counselling women at risk of developing breast cancer.
Risk assessment is important in decision-making with regard to screening programmes, prophylactic treatment and surveillance of women at risk.
Age, family history, menstrual history, parity and any previous surgery for breast disease are taken into account when assessing risk of developing cancer.
Alcohol increases the risk of breast cancer by increasing serum oestrogens concentration.
Future risk assessment models should include the mammographic and pathological results of benign breast disease.
16.1 The Concept of Risk Assessment in Benign Breast Lesions
Benign breast lesions can present symptomatically or mammographically detected through screening programmes. Risk assessment is important for both the clinician and the patient to assist in making decisions regarding screening, prophylactic treatment and surveillance. Because of widespread use of mammography, clinically silent benign lesions are now easily detected, leading to pathological assessment to exclude the presence of malignancy. Fine needle aspiration cytology, needle core biopsies and excision biopsies are the methods used to confirm benignity and allay patient anxiety. When a woman has a diagnosis of benign breast disease, more often than not she wishes to know what is the likelihood of the benign lesion predisposing to cancer at a later stage. With some conditions, such as fat necrosis, duct ectasia or hyalinised fibroadenoma, the clinician can reassure the patient confidently that there is no risk of developing cancer. However, some benign proliferative lesions confer an increased risk of subsequent malignancy, although in most cases the risk is very small. These conditions require careful explanation to the patient, not to alarm them but to encourage compliance with follow-up. The advantage of assessing the possible risk of progressing to malignancy in benign disease is that regular clinical and radiological follow-up can detect malignancy at an early, potentially curable stage.
Risk assessment is based on epidemiological studies, which combine demographic factors and pathological features of the benign lesions. However, accurate risk assessment of benign breast lesions can be hindered by the differences in terminology used in different publications as illustrated in the previous chapters with columnar cell lesions being a typical example. Until recently, most benign lesions were clinically and pathologically classified under the collective term ‘fibrocystic disease’ (Page et al. 1978; Love et al. 1982; Hutter et al. 1986). Although attempts have been made to standardise classification of benign lesions, inter- and intraobserver variation among pathologists when classifying atypical proliferations can reduce or increase the perceived risk of subsequent malignancy of a particular proliferative lesion.
Because of the above confounding factors, the actual incidence of benign breast disease is also variable, Ernster (1981). In a case-controlled study in Greater Boston, Cole et al. (1978) reported the incidence of benign breast disease to be 89.4 and 32.8 per 100,000 women for ‘fibrocystic disease’ and fibroadenoma, respectively. In Western Australia, Fleming et al. (1982) reported benign breast disease to be 420.2/100,000 women. The breast lesions in the latter study were classified into ‘benign mammary dysplasia’ and fibroadenoma. Prior to the introduction of the terminology which unified benign breast disease, the above conflicting figures would place the clinician in a difficult position as to which publication to believe. A woman with a mixed benign breast disease (busy breast) will no doubt have a higher risk of developing cancer than a woman with fibroadenoma.
16.2 Defining Risk and Related Terms
16.2.1 Definition of Risk
There is no readily available medical literature on the definition of risk and most useful information is in industry or technology-based publications. Fischhoff and Watson (1984) outlined in their essay the controversies around defining risk. They stated that ‘risk is a focal topic in the management of many activities and technologies’. For that management to be successful, an explicit and accepted definition of the term ‘risk’ is essential. Although this essay is on risk in general with regard to day-to-day industry and technology, it is applicable to assessing the risk for breast cancer or any other disease process because they further explain that ‘the choice of definition can affect the outcome of policy debates and allocation of resources’. They also highlight the technical distinction between objective and subjective risk, the former being a product of scientific research, public health statistics, experimental studies, epidemiological surveys and probabilistic risk analysis. In contrast, subjective risk is the public perception of that research. They also highlight the effects of comparing different scientists which can affect the relative riskiness of technologies. This is clearly illustrated in this book when different authors assess the relative risk of ADH, which ranges from 4 to 13 times that of the general public depending on which publication one reads (Dupont and Page 1985; Palli et al. 1991). However, in this paper Fischhoff and Watson do not provide the reader the definition of risk but highlight various factors to consider in assessing risk for any situation, which is also applicable to breast cancer. The factors the authors evaluated include the following:
(i)
Objective and subjective nature of the risk.
(ii)
Dimension of the risks, as risk is not a single consequence, e.g. in giving prophylactic tamoxifen to patients with ADH one should consider whether the risk of breast cancer outweighs the risk of endometrial cancer or death from thromboembolism.
(iii)
Taking into consideration summary statistics, which in benign breast disease several publications have done to evaluate the risk associated with different proliferative lesions of the breast.
(iv)
Willingness to count delayed effect of the risk, i.e. the evaluating of the risk is not bound by time as illustrated by the Gail Model which assesses risk in women aged 35–90 years of age. Furthermore, does circumventing one risk exposes individuals to another risk, e.g. use of prophylactic tamoxifen above.
(v)
The event should be one which threatens people’s health and safety sufficiently to raise concern, i.e. concern over developing breast cancer with risk of dying from the disease.
There is no definition of risk in the National Cancer Institute online dictionary of medical words. The online Oxford Dictionaries (British and World English) define risk as ‘a situation involving danger’. A better definition of risk is provided in online business dictionary which provides ‘a probability or threat of damage, injury, liability, loss or any other negative occurrence that is caused by external or internal vulnerabilities, and that may be avoided through pre-emptive action’ (Business Dictionary). The dictionary provides several examples of risks including that on cancer which is described as the consequence and probability of a hazardous event or phenomenon, i.e. the risk of developing cancer is estimated to be an incremental probability of developing cancer over a lifetime as a result of exposure to potential carcinogens.
16.2.2 NCI Dictionary of Cancer Terms Relative Risk
Risk assessment for breast cancer is divided into relative risk and absolute risk. The National Cancer Institute Dictionary of Medical Terms (NCI Dictionary of Cancer Terms) defines relative risk as ‘a measure of risk of a certain event happening in one group compared to the risk of the same event happening in another group’. In cancer research, relative risk is used in prospective (forward looking) studies such as cohort studies and clinical trials. A relative risk of one means there is no difference between two groups in terms of their risk of cancer based on whether or not they are exposed to a certain substance or factor or how they respond to two treatments being compared. A relative risk of greater than one or of less than one usually means that being exposed to a certain substance or factor either increases (relative risk greater than one) or decreases (relative risk less than one) the risk of cancer or that the treatments being compared do not have the same effect, also called the ‘risk ratio’.
Most relative risk estimates are from longitudinal studies and have been derived under the assumptions that the woman’s relative risk remains constant over time. However, it is possible that the relative risk of individual patients varies with age or the time since the original biopsy. To illustrate this point, Dupont and Page (1985, 1989) demonstrated that the relative risk of developing breast cancer in women with atypical hyperplasia and proliferative disease without atypia (PDWA) is greatest during the first 10 years of follow-up. Women with PDWA who remain disease-free after 10 years will no longer be at risk of breast cancer when compared with women of a similar age group without PDWA. Similarly, the relative risk of breast cancer in women with atypical hyperplasia is halved if they remain cancer-free for 10 years following the initial biopsy. This supports the notion that atypical hyperplasia may not be an obligate precursor of breast cancer and that the lesion may progress to breast cancer, remain static or regress over a period of time. However, the absolute risk of women with atypical hyperplasia remained constant over a period of 17 years of follow-up (Dupont and Page 1985). The clinical significance of this time-dependent analysis is that, depending on other factors, it will allow women with atypical hyperplasia to be closely followed up during the first 10 years of diagnosis. If the woman has not developed cancer at 10 years or more during follow-up, she can be reassured that her risk is now closer to that of the general population than it was at the time of the diagnosis of atypical hyperplasia, although the absolute risk per year has remained the same.
16.2.3 Absolute Risk
The National Cancer Institute Dictionary of Cancer Terms (NCI Dictionary of Cancer Terms) defines absolute risk as ‘a measure of risk of a certain event happening’. In cancer research, it is the likelihood that a person who is free of a specific type of cancer at a given age will develop that cancer over a certain period of time. For example, a woman of 35 years of age with no known risk factors for breast cancer has an absolute risk of developing breast cancer over a lifetime of 90 years of about 13.5 % meaning one out of every seven women will develop breast cancer by the age of 90.
Absolute risk is more informative than relative risk and can be determined over time intervals of 10–20 years when the corresponding relative risk estimates have been accurately determined (Dupont and Plummer 1996). To the patient, it is more important to know that the absolute risk of developing breast cancer if one had ADH is 10 % over a period of 10–15 years than to be told the relative risk of developing cancer is four times that of the general population. Although absolute values give a more accurate assessment of risk, they require lengthy follow-ups of large numbers of patients with similar risks (Dupont and Page 1989). For this reason, patient management is usually based on estimation of relative risk.
16.2.4 Risk Factors
For the probability of an event to occur, the individual must be exposed to a risk factor. This is another term which is not clearly defined in medical literature. As explained by Dr Brian Burt at the Department of Epidemiology (University of Michigan), the literature is unclear whether risk factors should be truly causal, i.e. a link in the aetiological chain or whether it is more peripherally associated with an outcome (Burt BA Definitions of risk). This statement is pertinent to benign breast lesions where it is not always clear whether the presence of a proliferation will directly metamorphose into breast cancer or it is just a marker of the likelihood that cancer will develop at some point in the woman’s lifetime.
In the dictionary of epidemiology, risk factor is defined as ‘an aspect of personal behaviour or lifestyle, an environmental exposure, or an inborn or inherited characteristic which on the basis of epidemiological evidence is known to be associated with health-related condition(s) considered important to prevent’ (Last 2001). Some examples cited as risk factors for cancer are age, a family history of certain cancers, use of tobacco products, being exposed to radiation or certain chemicals, infection with certain viruses or bacteria and certain genetic changes. This definition is wider and more inclusive when compared to the National Cancer Institute definition which defines risk factor as ‘something that increases the chance of developing a disease’.
An equally informative definition of risk factor is that provided by Dr Beck (1998) which states that ‘a risk factor is an environmental, behavioural or biological factor confirmed by temporal sequence, usually in longitudinal studies, which if present increases the probability of a disease occurring, and if absent reduces the probability’. Risk factors are part of the causal chain. Once disease occurs, removal of a risk factor may not result in a cure. Dr Beck (1998) explains that this definition is more specific as there is
(a)
Emphasis on the temporal sequence of exposure before outcome.
(b)
The acceptance that a risk factor is part of the causal chain.
(c)
The acceptance that risk factors are involved in disease onset, not necessarily in future progression or resolution.
The definition by Dr Beck is clearly applicable for ADH as risk factor for breast cancer in that
(a)
This proliferation has to be there before development of cancer.
(b)
ADH is considered part of causal chain to breast cancer because of the shared genetic alterations between ADH, DCIS and invasive cancer.
(c)
ADH is involved in onset but not the progression of cancer as other growth factors are involved.
16.2.5 Risk Marker
If some risk factors in breast cancer are part of the causal chain, this raises the question what is a risk marker? The dictionary of epidemiology (Last 2001) defines a risk marker as ‘an attribute or exposure that increases the probability of occurrence of disease or specified outcome’. This would apply to a lesion such as ALH which some consider to be a marker of rather than a causal factor of invasive breast cancer because the resultant breast cancer and can be either ductal or lobular and affect both breasts. In contrast, ADH is considered to be a causal link in the pathogenesis of breast cancer and is also a modifiable risk factor, i.e. a determinant that can be modified by intervention, thereby reducing the probability of disease (Dictionary of Epidemiology, Last 2001). There is evidence that prescribing prophylactic tamoxifen to women with ADH reduces the risk of developing cancer, however, the same applies to ALH (Chuba et al. 2005).
16.3 General Risk Factors for Breast Cancer
16.3.1 Genetic Factors
Assessment of risk factors for breast cancer can be divided into those individuals who have a familial predisposition because they carry a genetic mutation such as BRCA1 and BRCA2 and those without a mutation (Parmagiani et al. 1996). Five to ten percent of breast cancer occurs as a result of genetic predisposition caused by a mutation in a single cancer susceptibility gene which confers considerable risk (Vogel and Bevers 2003). Features of familial breast cancer include
Breast cancer in two or more relatives from the same lineage
Early age at diagnosis (<50 years of age)
Bilateral disease
Multiple primary tumours (breast and ovarian)
Group of features consistent with a genetic syndrome
Male breast cancer
Ashkenazi Jewish ancestry
A relative with a mutation in a known cancer susceptibility gene.
BRCA1 gene and BRCA2 genes which predispose to hereditary breast and ovarian cancer account for 81 % and 14 % of hereditary breast cancer, respectively (Ford et al. 1998). Other genetic syndromes associated with familial breast cancer include Cowden syndrome, Li-Fraumeni syndrome, ataxia-telangiectasia heterozygotes and Peutz–Jeghers syndrome (Vogel and Bevers 2003). The UK NICE recently recommended the use of tamoxifen for chemoprevention in women with high risk of developing cancer due to familial predisposition (June 2013). In addition to the above risk factors the NICE Guidelines include
Sarcoma in a relative younger than 45 years of age
Glioma or childhood adrenocortical carcinomas
Complicated patterns of multiple cancers at a young age
Very strong paternal history (for relatives diagnosed at younger than 60 years of age on the father’s side of the family)
16.3.2 Hormonal Factors
Hormonal changes play an important role and reduce or increase the risk of breast cancer in an individual. Early menarche (<12 years), late menopause (>55 years), nulliparity or first born child after 30 years and current use of oral contraceptives are associated with increased risk of breast cancer (Vogel and Bevers 2003). The risk is due to the high levels of circulating oestrogens.
Earlier studies published conflicting evidence as to whether hormone replacement therapy (HRT) is associated with an increased risk of breast cancer or not (Dupont et al. 1999). The presence of oestrogen and progesterone receptors in the breast tissue makes it sensitive to circulating hormonal therapy. A meta-analysis of 51 international case control and cohort studies found no appreciable increased risk of breast cancer in women on short-term oestrogen replacement therapy of less than 5 years, (Manson and Martin 2001). However, long-term use of oestrogen replacement therapy (>5 years) was associated with an increased risk of 35 %. A separate study by the Writing Group for Women’s Health Initiative Investigators, reported an increased risk of 26 % in women on combination HRT (2002).
A multicentre study involving over one million women, in which 9,364 women developed cancer and 637 women died from breast cancer, Beral and the Million Women Study Collaborators (2003) observed that current users of HRT were more likely than ‘never users’ to develop cancer (adjusted RR = 1.66, 95 % CI = 1.58−1.75, P < 0.0001) and to die from breast cancer (RR = 1.22, 95 % CI = 1.00−1.48, P = 0.05). The risk of breast cancer was increased much more with combined oestrogen and progesterone preparations than with oestrogen only and other preparations. Long duration of HRT use, up to 10 years, was also associated with an increased risk. Based on these findings, it may be clinically advantageous to assess the parenchymal density of the women before they commence HRT and offer shorter interval screening periods for those with a significant increase in parenchymal density while on HRT. This may detect women with early cancer when this is potentially curable. Selective oestrogen-receptor modulators (SERMs) such as tamoxifen and raloxifene may be an alternative to HRT for women at risk for breast cancer (Vogel et al. 2010).
16.3.3 Alcohol as a Risk Factor
There is accumulating evidence that alcohol intake is associated with increased risk of breast cancer (Vogel and Bevers 2003; Longnecker 1994; Longnecker et al. 1995; Rosenberg et al. 1993). The following mechanisms have been implicated:
Induces increased levels of circulating oestrogen
Stimulates hepatic metabolism of carcinogens such as acetaldehyde
Facilitates transport of carcinogens into the breast tissue
Stimulates pituitary production of prolactin
Modulates cell membrane integrity with an effect on carcinogenesis
Aids production of cytotoxic protein products
Impairs immune surveillance
Interferes with DNA repair
Promotes production of toxic congeners
Increases exposure to toxic oxidants
Reduces intake and bioavailability of protective nutrients
Coutelle and colleagues (2004) demonstrated that oral intake of alcohol in women with alcohol dehydrogenase 1C*1 allele, which rapidly metabolises alcohol to produce increased levels of acetaldehyde have increased levels of serum estradiol. The women with this alcohol dehydrogenase genotype were 1.8 times more at risk of developing breast cancer than women without the genotype (95 % CI 1.431–2.330, p < 0.001). Thus, individuals with this genotype increase their risk of breast cancer if alcohol is consumed in excess, resulting in increased blood oestrogen concentration.
In a previous study, Wright et al. (1999) proposed that metabolism of alcohol produced reactive oxygen species which induce carcinogenic mutations and damage to DNA predisposing to breast cancer. The amount of alcohol consumed is directly proportional to the risk of breast cancer. Drinking 10 g of alcohol (one drink daily) is associated with relative risk of 1.09 when compared to non-drinkers. Increasing alcohol consumption to 30–60 g/day (2–5 drinks) increased the relative risk of breast cancer to 1.41 when compared to non-drinkers (Smith-Warner et al. 1998). Alcohol consumption in individuals with family history of breast cancer increases the RR of developing breast cancer to 2.45 (Vachon et al. 2001). The risk is similar for beer, wine and liquor (Smith-Warner et al. 1998).
16.3.4 Other Risk Factors for Breast Cancer
Other factors associated with increased risk of breast cancer are old age and high body mass index (BMI), but there is no evidence that diet and exercise influence the occurrence of breast cancer (Vogel and Bevers 2003). As already illustrated in the previous chapters, different types of benign proliferative disease confer varying degrees of risk of subsequent breast cancer and these will be summarised in this chapter.
16.4 Radiological Risk Factors
16.4.1 Wolfe’s Breast Parenchymal Patterns
John Wolfe highlighted the concept of breast cancer risk based on mammographic parenchymal patterns, and most of the publications on this subject were in 1976. Age, pregnancy, thyroid hormone levels, endogenous and exogenous hormones can influence breast parenchymal appearances. Wolfe classified the appearance of the breast parenchyma into four categories irrespective of vasculature, presence or absence of masses, calcifications, parity, family history of breast cancer or age of the patients. He noted four breast parenchymal patterns, which he classified as follows:
N1 = Normal, consisting of mostly fat (fatty breasts).
P1 = Consists of mostly fat but in the subareolar area or other quadrant, there is a linear pattern of prominent ducts.
P2 = Breast parenchymal with more prominent duct pattern. The ducts exhibit a triangular disposition in the central portion of the breast. This pattern involves more than a quarter of the breast volume.
DY (for ‘dysplasia’) = Generalised increase in the density of the breast parenchyma with or without prominent ducts.
The exact histological features of ‘dysplastic’ breast tissue are not clear from Wolfe’s original study (Wolfe 1976a). However, Wellings and Wolfe (1978) carried out a radiological and pathological correlation of tissue from the different parenchymal patterns. This included subgross analysis of the excised breast tissue. The histological features of the different parenchymal patterns were reported as follows:
N1 = Fat, delicate vascular elements, fibrous bands containing normal lobules
P1 = Increased periductal and perilobular fibrosis. The lobules were normal
P2 = Increase in fibrosis with ‘moderate epithelial atypia’
DY = Marked fibrosis. ‘Overt atypical’ lobules and incidental cysts present.
16.4.2 Parenchymal Patterns and Risk of Breast Cancer
When Wolfe (1976b) assessed the different radiological parenchymal patterns in relation to the risk of breast cancer, the incidence of detecting cancer in the N1, P1, P2 and DY parenchymal patterns was 0.1, 0.4, 1.7 and 2.2 %, respectively, indicating that dense breast carried the highest risk of harbouring malignancy. Based on these results, the risks of the parenchymal patterns were assessed as follows: N1 = minimal risk, P1 = moderate risk, P2 = significant risk, DY = highest risk.
In a subsequent report, Wolfe (1976c) followed up 995 women who had undergone mammography over a period of 15 years (range 5–15 years). All women had presented symptomatically (age range 30–49 years). The study reported that breasts initially classified as N1, P1 or P2 rarely changed their parenchymal density with age. In contrast, breasts classified as DY mostly regressed to P2, which is still a high-risk category, with some breasts regressing to P1 or N1. These changes occurred between 35 and 50 years. In a similar but separate study, Wolfe et al. (1987) reported that most breast cancers occurred in the P2 breast, with decreasing frequency in the DY, P1 and N1 parenchymal patterns. In this case-controlled study of 160 women who had presented symptomatically (mean age 53 years), the RR of developing breast cancer was 3.3 in women who had P2 or DY patterns when compared with women who had N1/P1 patterns. Black women had a higher risk of 5.2 when compared with white women with an RR of 2.3 for the P2 and DY parenchymal patterns. Women younger than 53 years had a lower RR of 2.8 compared with 3.8 for women older than 53 years if their mammograms exhibited the P2 and DY parenchymal patterns.
In a separate study, Saftlas and colleagues (1991) assessed parenchymal density in women undergoing screening mammography. The authors reviewed mammograms that were taken 4 years prior to diagnosis of breast cancer to determine whether increased mammographic density was predictive of breast cancer risk. The median ages of the women were 54 and 53 for the study cases (n = 266) and controls (n = 301), respectively. This study applied planimetry to assess the percentage of mammographic density and this had a better predictive value than Wolfe’s parenchymal patterns. The odds ratios (OR) of breast cancer increased steadily with increasing breast density (P < 0.0001). The breast cancer OR were 1.7 for women with 5–24.9 % parenchymal density, 2.5 for women with 25–44.9 % parenchymal density, 3.8 for women with 45–64.9 % parenchymal density and 4.3 for women with densities over 65 %. These OR were irrespective of other breast cancer risk factors such as family history of cancer or age at first parity.
Byrne et al. (2001) assessed the histological features of benign breast disease and mammographic density of the breast in a case-controlled study of 347 women who developed breast cancer and 410 women without breast cancer. Adjusting for mammographic density, the OR for subsequent cancer for atypical hyperplasia was 2.1. In contrast, adjusting for benign breast histology, the OR for increased breast density of >75 % was 3.8. Women with non-proliferative benign breast disease and increased breast density of >75 % had an OR of 5.8, whereas women with breast density of less than 50 % and atypical hyperplasia had an OR of 4.1.
Previously, Friedenreich and colleagues (2000) assessed the mammographic density of fibroglandular tissue as a risk factor for subsequent malignancy in the screening age group. They assessed women who attended the Alberta Screening Programme in Canada. The women were asymptomatic, aged between 50 and 69 years and attended for breast screening biannually. The aim of the study was to determine whether women with histologically proven benign breast disease had a radiologically identifiable increase in breast tissue density. The study compared 165 biopsy-proven benign diseases with 217 women who had no histological evidence of proliferative breast disease. The proliferative benign disease was classified into multiple papillomas, radial complex sclerosing lesion, nodular adenosis, sclerosing adenosis, usual hyperplasia (moderate or florid), ADH and ALH. The mammographic content of fibroglandular tissue was assessed as follows: (1) fatty, (2) >0 – <25 %, (3) 25–50 %, (4) 50–75 %, (5) >75 %. The women also responded to a questionnaire on demographic factors, which included smoking and dietary habits, menstrual and reproductive history, physical fitness, hormone usage and family history of benign proliferative breast disease. When all the demographic and radiological features were assessed, the authors found that women with increased fibroglandular density on mammography of >25 % had an increase in benign proliferative disease when compared with women with less than 25 % of fibroglandular tissue (OR = 1.91; 95 % CI = 1.24−2.94). Most studies that assessed benign breast disease for the potential risk of malignancy were based on symptomatic patients. The study by Friedenreich and colleagues should pave the way for prospective follow-up studies, which should combine demographics, radiological and histological features of benign proliferative disease to accurately prognosticate the risk factors in the screening age group. In a separate study, Boyd et al. (2000) reported a high prevalence of benign epithelial hyperplasias associated with increased mammographic densities of over 75 %. The benign epithelial hyperplasias were in turn risk factors for malignancy. The findings were comparable to those of Saftlas and colleagues (1991).
The association of breast cancer risk with increased breast tissue density has not been universally supported by other investigators (Mendell et al. 1977; Moskowitz et al. 1980; Arthur et al. 1990). In the UK Trial for Early Detection of Breast Cancer in Nottingham, Arthur and co-workers (1990) examined breast tissue from 119 women with a histological diagnosis of fibrocystic change with epithelial hyperplasia or in situ carcinoma. Women attending routine breast cancer screening were used as controls. The Nottingham study reported that the Wolfe patterns were related to the distribution of fibrous and adipose tissue in the breast interlobular stroma and appeared to have no relationship to the epithelial parenchymal content. Based on these findings, the authors concluded that radiological densities of P2 and DY patterns did not correspond to high-risk epithelial proliferations.
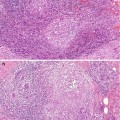
Despite the variation in the reports regarding the significance of breast tissue density as a risk factor for breast cancer, there is a growing evidence that increased breast tissue density may be a risk factor for subsequent malignancy. It is not clear whether the increased risk of malignancy associated with dense breasts is due to masking of small cancer because mammographic interpretation of dense breast is difficult, or whether mammographic density is an independent risk factor (Fig. 16.1). However, there is evidence that including mammographic density as a risk factor minimally improves the predictive accuracy of the Gail Model (Tice et al. 2006).
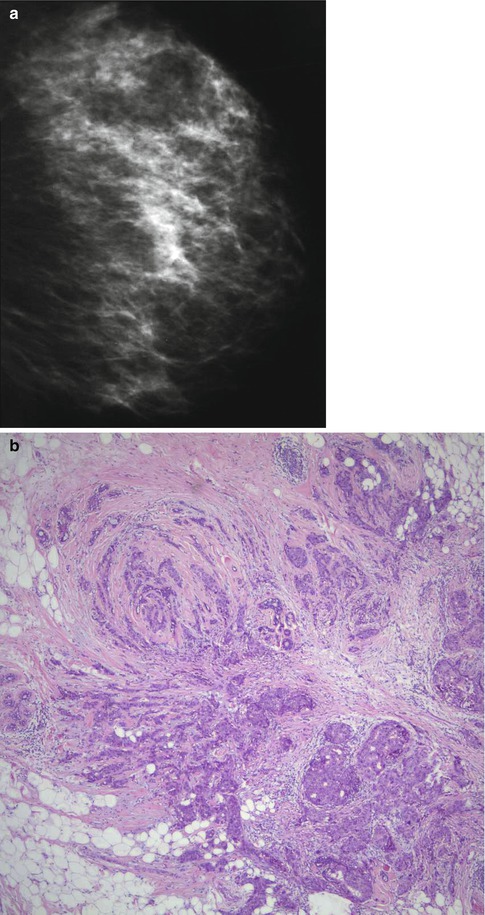
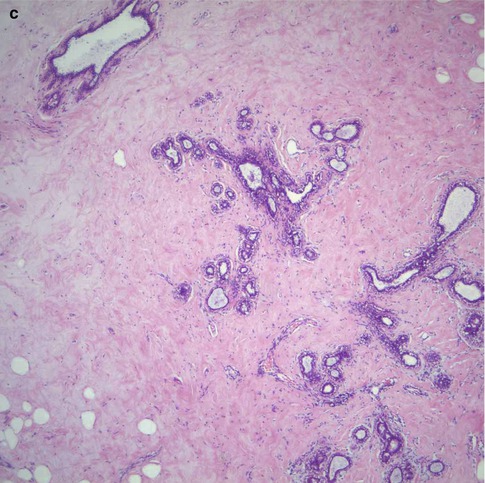


Fig. 16.1
(a) This mammogram illustrates moderately dense breast in a 45-year-old patient who presented symptomatically with ill-defined tender lumpiness in the left breast. There is no discrete lesion in mammograph. (b) Although there was no definite lesion on mammography, the patient requested to have the ‘lump’ excised. An incidental grade 3 carcinoma was identified on histology. (c) The rest of the breast tissue showed widespread fibrosis with atrophic duct–lobular units with areas of fat infiltration. There was no significant benign epithelial proliferation to explain the increased mammographic density
16.4.3 Effect of Hormone Replacement Therapy on Breast Density