Key Words
deep vein thrombosis, clinical management, algorithms, deep venous thrombosis risk assessment, imaging protocols
Introduction
Duplex sonography can effectively diagnose the presence of acute and chronic venous thrombosis in the extremity veins. Most commonly, duplex sonography is used when acute deep vein thrombosis is suspected, but it is also a reliable means for determining the extent of chronic venous disease and the accompanying physiologic alterations in venous hemodynamics. Performance of the diagnostic venous ultrasound examination is also tailored to current approaches in patient management and the availability of various treatments. Both these aspects will be covered in the following pages.
Acute Deep Vein Thrombosis Etiology and Risk Factors
Venous thromboembolic (VTE) disease includes both deep vein thrombosis (DVT) and pulmonary embolism (PE) because they represent different aspects of the same disease process. Suspected VTE is the most common indication for the clinical evaluation of the extremity veins. Although a comprehensive review of VTE is beyond the scope of this chapter, a brief review of risk factors and conditions fostering the development of VTE is given. The annual incidence of VTE in the United States is estimated at over 2.5 million cases. Roughly 25% of untreated patients with DVT will sustain a nonfatal PE. Moreover, without treatment, PE is associated with mortality of approximately 30%. A myriad of clinical conditions increases the risk of incident venous thrombosis. This susceptibility to develop venous thrombosis was first described in 1865 as Virchow’s triad: (1) venous stasis, (2) endothelial damage, and (3) hypercoagulability.
Venous stasis can occur in any situation of prolonged immobility. It may also be promoted by any situation where venous return to the heart is obstructed by compression of the vein lumen as can be seen in cases of pelvic tumors.
Endothelial damage includes direct trauma to the vein through puncture of the lumen. It can also occur in cases of trauma when shearing forces are applied to the vein wall and the endothelial layer is disrupted. This exposes the underlying collagen-rich tissues to blood.
Thrombus formation occurs through the activation of enzymatic reactions in the intrinsic and tissue factor coagulation pathways ( Fig. 20.1 ). This leads to thrombin formation via the prothrombin enzyme complex. The thrombomodulin-protein C system primarily limits coagulation, while the fibrinolytic system further limits fibrin deposition. This homeostatic system is continuously active and balances activation and inhibition of coagulation and fibrinolysis. The predisposition to thrombus formation results either from nonreversible (genetic factors, age) or reversible (acquired) prothrombotic conditions ( Table 20.1 ).

| Genetic Risk Factors | Acquired Risk Factors | Environmental |
|---|---|---|
| Elevated factor VIII, IX, or XI | Age | Immobilization including travel |
| Factor V Leiden | Prior venous thrombosis or pulmonary embolism | Major surgery within 3 months |
| Primary hyperhomocysteinemia | Oral contraception and hormone replacement therapy | Oral contraception and hormone replacement therapy |
| Protein S deficiency | Malignancy | Central venous catheters |
| Antithrombin III deficiency | Obesity (BMI ≥30 kg/m 2 ) | Pregnancy and postpartum state |
| Protein C deficiency | Cigarette smoking | Trauma |
| Non-O ABO blood group | Hypertension | Chemotherapy |
| Dysfibrinogenemia | Secondary hyperhomocysteinemia | |
| Acquired antiphospholipid syndrome | ||
| Congestive heart failure | ||
| Myeloproliferative disorders | ||
| Nephrotic syndrome | ||
| Inflammatory bowel disease | ||
| Sickle cell anemia | ||
| Marked leukocytosis in acute leukemia | ||
| Infectious (e.g., sepsis, HIV) |
Inherited prothrombotic disease states have been described with increasing frequency over the past 30 years. This category of venous thrombosis is considered nonreversible and, as such, the patient keeps his/her increased risk of developing venous thromboembolic disease throughout life.
Antithrombin III deficiency was the first reported congenital thrombotic condition. It is transmitted as an autosomal dominant pattern with a prevalence of 1 : 5000. Isolated spontaneous thrombosis has been described with this condition. This deficiency lowers the threshold for the development of DVT in the presence of precipitating circumstances, such as trauma, pregnancy, and surgical procedures.
Protein C and protein S are vitamin K–dependent cofactors that facilitate degradation of activated factor V. Deficiencies therefore predispose to thrombosis. Congenital deficiencies of these factors are well described. Because these proteins are synthesized in the liver, acquired deficiencies may also occur from variations in liver function as well as with dietary changes. Protein C or S deficiency confers a roughly sevenfold increased risk of developing venous thrombosis. Resistance to activated protein C is also known as factor V Leiden. This disorder results from a point mutation in the factor V gene, rendering activated factor V resistant to degradation by activated protein C. It is present in 12% to 33% of patients with spontaneous VTE, making it the most common inherited hypercoagulable condition.
Factor II (prothrombin) G20210A is a mutation seen in 2% to 3% of individuals, predominantly those of European descent. It can increase the risk of VTE by as much as 2.8 times compared with individuals without this mutation.
Primary hyperhomocysteinemia increases risk for VTE, along with the development of premature atherosclerosis. Serum elevation of coagulation factors VIII, IX, and XI have been shown to confer elevated risk for venous thrombosis in the Leiden thrombophilia study. Factor IX and XI levels greater than the 90th percentile, respectively, confer a 2.5-fold and 2.2-fold increased risk of VTE. Dysfibrogenemias and hypofibrinolysis impair the steps involved in the generation, cross-linkage, and breakdown of fibrin. Bleeding diathesis, as well as VTE, has been described with this condition.
Acquired prothrombotic states are more numerous than inherited states. Clinical conditions that predispose to VTE are listed in Table 20.1 . Several of these conditions are briefly considered. Pregnancy and the postpartum period increase VTE risk compared with the nonpregnant state. PE is a leading cause of maternal death after childbirth, with one fatal PE per 100,000 births. Oral contraceptives and hormone replacement therapy can increase the risk of VTE in premenopausal and postmenopausal women. Lidegaard and colleagues reported a prevalence of VTE in women receiving oral contraceptives of one to three in 10,000. Women receiving hormone replacement therapy have a twofold increased risk of VTE with rates depending on the type of contraceptive and a greater risk at the onset of therapy. Antiphospholipid antibody syndrome is an acquired condition due to the presence of either the lupus anticoagulant antibody or anticardiolipin antibodies. Overall, the syndrome can be identified in 1% to 5% of the population. Among those with positive titers for the lupus anticoagulant, the risk of developing VTE is 6% to 8%. Patients with anticardiolipin antibody titers greater than the 95th percentile have a 5.3-fold increased risk of developing VTE.
Other risk factors for VTE that are often ignored include age and elevated body mass index (BMI). VTE is uncommon in children but increases progressively with age after adolescence. Obesity, defined as an increased BMI ≥30 kg/m 2 is associated with a 2 to 3 times increased risk of DVT. Trauma and concurrent malignancy remain two major sources of provoked episodes of deep vein thrombosis.
Wells score and D-dimer levels
An important consideration for the triage of patients is the application of the Wells score ( Table 20.2 ). This clinical prediction rule is a useful guide for determining the need to perform a diagnostic imaging test, most often venous ultrasound, given an a priori likelihood that the patient has deep vein thrombosis. Once calculated, the Wells score can be used to estimate the likelihood of DVT: a low risk is a score of 0 or less, an intermediate probability is a score of 1 or 2, and a high probability is a score of 3 or more.
| Clinical Characteristics | Points |
|---|---|
| Active cancer (patient receiving treatment for cancer within the previous 6 months or currently receiving palliative treatment) | +1 |
| Paralysis, paresis, or recent plaster immobilization of the lower extremities | +1 |
| Recently bedridden for 3 days or more or major surgery within the previous 12 weeks requiring general or regional anesthesia | +1 |
| Localized tenderness along the distribution of the deep venous system | +1 |
| Entire leg is swollen | +1 |
| Calf swelling at least 3 cm larger than that on the asymptomatic side (measured 10 cm below tibial tuberosity) | +1 |
| Pitting edema confined to the symptomatic leg | +1 |
| Collateral superficial veins (nonvaricose) | +1 |
| Previously documented DVT | +1 |
| Alternative diagnosis at least as likely as DVT | –2 |
The D-dimer test detects the presence of breakdown products of linked fibrin, the main constituent of thrombus, due to in vivo thrombolysis. Although the test is very sensitive for the presence of thrombus, it is not specific. False positives can be due to trauma, malignancy, recent surgery, pregnancy, liver disease, or renal disease. In addition, the sensitivity of the test depends on the method used. The most sensitive is the ELISA (enzyme-linked immunosorbent assay) method, a test that is time-consuming, has an overall sensitivity of 95%, and is considered abnormal at a level above 500 µg/L. Other, more rapidly processed D-dimer tests show some heterogeneity in sensitivity and cut points.
The D-dimer test is often combined with the Wells score to help triage patients requiring compression ultrasound. For example, venous imaging may not be performed if a patient has both a low Wells score and a negative D-dimer test. This situation can occur in up to 40% of the outpatient population.
Anticoagulation and Thrombolysis in the Management of Venous Thrombosis
Overview
The management of patients with suspected DVT may affect the protocol implemented during ultrasound imaging of the extremity veins. Anticoagulation with an agent that offers a lower risk of bleeding than unfractionated heparin can justify postponing a venous ultrasound examination requested in the middle of the night to the next morning. The availability of new oral agents that permit effective treatment of calf vein DVT without excess risk of bleeding may help justify imaging of the calf veins. Low-risk anticoagulation being performed in the outpatient setting may necessitate a more quantitative evaluation of thrombus burden in order to help triage patients to outpatient or inpatient treatment.
Heparin
Anticoagulation with intravenous (unfractionated) heparin has been the gold standard for the initial management of DVT for decades (see Fig. 20.1 ). Heparin has an antifactor Xa effect. Heparin potentiates the action of antithrombin III, thereby preventing additional thrombus formation and permitting endogenous fibrinolysis. It also has an effect on inflammation (the “itis” in thrombophlebitis). Use of low molecular weight (fractionated) heparin compounds (enoxaparin [Lovenox] or dalteparin [Fragmin]) has decreased the risk of bleeding while keeping most of the benefits of unfractionated heparin. Low molecular weight heparins can be administered subcutaneously once or twice a day and do not require monitoring of the aPTT (activated partial thromboplastin time). They have permitted the empirical treatment of patients with suspected DVT while waiting for definite diagnostic testing ( Fig. 20.2 ).

In the absence of a contraindication to anticoagulation, prompt institution of heparin therapy is indicated for patients with either confirmed DVT (through imaging techniques) or for patients in whom a moderate or high clinical suspicion of DVT exists (see Fig. 20.2 ). Low molecular weight heparin is preferred if confirmatory venous ultrasound is not immediately available.
Vitamin K antagonists
Oral warfarin therapy is started while therapeutic heparin anticoagulation is being administered, usually over 5 days. Warfarin dosing is guided by measuring the International Normalized Ratio (INR), a reflection of the inhibition of vitamin K–dependent cofactors (see Fig. 20.1 ). Although the target INR will vary depending on the clinical circumstance, it is important to understand that early elevations in the INR (1 to 3 days after institution of warfarin therapy (with an INR target of 2 to 3) usually result from inhibition of factor VII because of its short half-life. Effective anticoagulation depends on the depletion of factor II (thrombin) and typically requires about 3 to 4 days of warfarin therapy to achieve a stable INR. This leads to a transition period of approximately 5 days. Therapy with oral warfarin in the absence of heparin anticoagulation should be avoided, as days will pass before anticoagulation is adequate, leaving the patient unprotected against PE; moreover, warfarin therapy in the absence of heparin anticoagulation may paradoxically intensify hypercoagulability and predispose to recurrent DVT.
The duration of anticoagulation varies with the clinical scenario. In general, for initial cases of uncomplicated DVT, 3 months of anticoagulation is recommended. Inherited and acquired procoagulant states, and cases of recurrent DVT may require longer anticoagulation therapy, typically 6 months. In some cases of recurrent VTE and irreversible risk factors, lifelong anticoagulation may be recommended.
New therapeutic agents
Oral agents not based on suppression of vitamin K–dependent coagulation factors are increasingly used to treat DVT. They are referred to as the direct oral anticoagulants (DOACs). There are currently two classes of these medications available: direct factor Xa inhibitors and direct factor IIa inhibitors. Both are considered acceptable substitutes to vitamin K–inhibitors for the treatment of deep vein thrombosis. Overall, these agents have a lower risk of bleeding than warfarin. The factor IIa (direct thrombin) inhibitors require coverage with heparin for the first 5 days in the same way as warfarin. The Xa inhibitors rivaroxaban and apixaban can be administered on the day of diagnosis without the need for 5 days of heparin treatment. Edoxaban has only been administered after heparin coverage in the Hokusai trial.
Thrombolysis
Thrombolysis is not commonly used to treat patients with DVT, but there are situations in which it should be considered. Thrombolysis may be used to treat patients with extensive iliofemoral venous thrombosis and severe symptoms, where the risk of development of the postthrombotic syndrome is high. The prevalence and severity of the postthrombotic syndrome is believed to be decreased if rapid thrombolysis is achieved. However, the substantial proportion of patients with contraindications to thrombolysis and the associated increase in major bleeding episodes severely limit the use of thrombolytic therapy in more peripherally located DVT.
Thrombolysis is a therapeutic consideration in patients with upper extremity effort associated venous thrombosis, especially if correction of the underlying abnormality is to be attempted.
Guidelines for the management of acute deep vein thrombosis
Patients may be selected for ultrasound imaging of suspected DVT based on published guidelines. For example, the American College of Chest Physicians (ACCP) generates guidelines based on the review of the current literature. The last set of complete guidelines, the 9th set, was published in 2012. Some of the topics were reviewed in 2016 and published as the 10th set. Ultrasound imaging strategies for the diagnosis and management of DVT will be discussed in the next few sections. Whenever possible, reference will be made to these guidelines.
- •
Well-recognized risk factors for DVT include stasis, endothelial damage, and hypercoagulopathy (Virchow’s triad).
- •
Hypercoagulopathy may be associated with reversible risk factors such as pregnancy or be caused by genetic factors.
- •
The Wells score assigns a level of likelihood for the presence of DVT by considering predetermined risk factors.
- •
The D-dimer test is a very sensitive but nonspecific method for determining the likelihood of DVT.
- •
The D-dimer test and the Wells score can be combined to triage patients for further ultrasound imaging.
- •
Available anticoagulants with lower risk of bleeding than heparin or warfarin may impact the imaging protocol by:
- •
inclusion of the calf veins in the imaging protocol
- •
permitting overnight anticoagulation of patients when ultrasound imaging is not available
- •
facilitating outpatient management of patients with DVT
- •
- •
Although thrombolysis can dissolve thrombus quickly and decrease the incidence of chronic venous disease, it is preferably used in cases of iliofemoral DVT because of the risk of bleeding.
- •
Use of guidelines, such as those generated by the American College of Chest Physicians, may affect patient selection for ultrasound imaging and may modify the imaging protocol.
Acute Deep Vein Thrombosis of Specific Extremity Veins
Isolated calf vein/distal deep vein thrombosis
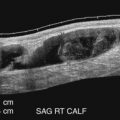
Although the term calf vein thrombosis is commonly used, another way of classifying the location of the veins is to refer to isolated distal deep vein thrombosis (IDDVT). This allows for the fact that the below-the-knee popliteal vein is a proximal vein. In this case, extension of thrombus from the muscular (gastrocnemius) or the axial (tibial and peroneal) veins to the popliteal vein is an indication for anticoagulation. Most lower extremity DVTs originate in the deep veins of the calf, although acute thrombus can form anywhere in the venous system. The soleal sinuses of the calf are thought to be the most common site of origin of DVT and are the most common site of residual thrombus in patients dying of pulmonary embolism. Untreated calf vein thrombus can progress into the popliteal and femoral veins in up to 30% of cases, whereas this risk is drastically reduced by anticoagulation. Once the thrombus propagates into the popliteal or femoral vein, therapeutic anticoagulation is needed to decrease the likelihood of pulmonary embolism. However, the clinical importance of isolated distal DVT remains uncertain. Abundant literature has been published, but much of it is contradictory.
The prevalence of isolated distal DVT in specific patient groups is difficult to establish because many studies include mixed patient populations and a variety of diagnostic techniques. For example, a study by Atri and colleagues attempted to separate patient populations by examining an asymptomatic postoperative high-risk group and a symptomatic ambulatory group. In the asymptomatic postoperative group, 20% of patients were found to have isolated calf DVT; in the symptomatic ambulatory group, the prevalence was 30%. This and similar studies indicate that although it is difficult to establish the incidence precisely, isolated calf DVT is not uncommon.
Once started, calf DVT can propagate to the popliteal vein and more proximal veins. There are two important questions that need addressing: (1) what is the likelihood that a distal DVT will propagate, and (2) are there indicators of possible thrombus propagation? The reported frequency of calf DVT propagation varies markedly. In postoperative patients, the reported rate of propagation varies from 6% to 34%. Although it is not possible to identify thrombi that are likely to propagate and distinguish them from those that do not, there are certain markers that suggest a higher risk of propagation: (1) length of thrombus 5 cm or more, (2) diameter of the involved vein of 7 mm or more, and (3) involvement of multiple veins.
Some authors argue that few, if any, significant pulmonary emboli arise from isolated calf DVT and therefore anticoagulation is unnecessary in the absence of demonstrable propagation. Other investigators have reported that the prevalence of PE in patients with calf-only thrombi is about 6.9% and that distal vein thrombi, especially in the soleal veins, are the most common finding in patients dying of acute pulmonary embolism. These studies are limited by the fact that they are not prospective. Meta-analysis of the importance of calf vein thrombosis have provided indeterminate results.
Studies have tended to show an advantage in treating distal vein DVT. However, recent controlled trials have shown a trade-off between a minimal decrease in DVT extension and PE versus an increased risk of bleeding while undergoing therapy.
Although there is no strong consensus over the prevalence of isolated calf DVT, current clinical practice guidelines give consideration to treating calf vein DVT because of its propensity to progress, the underlying risk of PE, and the likelihood of the postthrombotic syndrome.
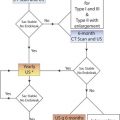
Recent ACCP guidelines provide a somewhat complex strategy for dealing with suspected distal DVT. The primary recommendation is not to look at the calf veins during the lower-extremity ultrasound examination if the D-dimer levels are not elevated ( Fig. 20.3 ). Depending on the clinical assessment, repeat above-the-knee ultrasound is considered in a subset of the population. The argument in favor of this strategy is a risk-benefit analysis taking into consideration the cost of treatment, the risk of bleeding when on treatment, and the low likelihood of calf-vein extension in most patients. A repeat above-the-knee (popliteal and common femoral veins) ultrasound performed at 7 days following a first negative above-the-knee venous ultrasound also has a false negative rate of 0.6% to 0.9%. The guidelines also recognize that the false-negative rate, in essence the 3 months recurrence rate of VTE after a negative complete lower extremity venous ultrasound, is very low and estimated within the range of 0.1% to 1.25%. However, if a complete lower extremity venous ultrasound identifies a calf-vein DVT, there are two strategies ( Fig. 20.4 ): (1) treat for 3 months, and (2) repeat the venous ultrasound for evidence of extension at 5 to 7 days. In addition to a positive D-dimer, the following imaging criteria are believed to indicate a higher likelihood of IDDVT extension and can justify anticoagulation: (1) thrombus length of 5 cm or more, (2) diameter of the involved vein of 7 mm or greater, and (3) involvement of multiple veins. The currently recommended duration of anticoagulation for calf-vein DVT is 3 months, although prior guidelines had recommended 6 weeks.
- •
Calf-vein DVT is, in essence, distal DVT, taking into consideration that a portion of the popliteal vein is below the knee. Popliteal vein involvement by DVT is evidence of proximal DVT.
- •
The spread of thrombus from the calf veins to the popliteal vein is an indication for full anticoagulation.
- •
The likelihood of calf-vein thrombus extension to the proximal veins varies between 6% and 34%. A plausible set of indicators for the spread of calf vein DVT include:
- •
length of thrombus 5 cm or more
- •
diameter of the involved vein of 7 mm or more
- •
involvement of multiple veins
- •
- •
Arguments have been made not to image the calf veins in cases with a clinically low risk for DVT.
- •
Localized pain is an indication for direct imaging of the calf veins with the location identified by the patient.
- •
Guidelines suggest that if the calf veins are not imaged, repeat common femoral and popliteal vein ultrasound at 7 days can exclude the spread of thrombus.
- •
If thrombus is identified in the calf veins, monitoring for possible progression by 5 to 7 days while withholding anticoagulation is a possible strategy.


Femoropopliteal vein thrombosis
Above-the-knee or proximal deep vein thrombosis is a more serious clinical problem than isolated calf (distal) DVT. The risk of PE is greater, thus absolutely requiring therapeutic anticoagulation. For patients in whom anticoagulation is contraindicated, vena cava filter placement is a therapeutic alternative. The clinical presentation of DVT may change appreciably as thrombus propagates more proximally. The calf is usually painful when the lower femoral vein or upper popliteal vein are involved. Swelling and warmth are typically evident on physical examination. With thrombus extension into the common femoral or iliac vein, the leg becomes painful and the patient will complain that it feels tight. Swelling may extend to the inguinal ligament, and the patient may be tender over the course of the veins, particularly in the inguinal region.
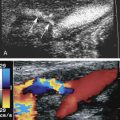
Duplex sonography is accepted as the definitive diagnostic test in patients suspected of having femoropopliteal DVT (see Chapter 19 ). Duplex sonography easily permits visualization of occlusive as well as partially occlusive thrombi. The presence of proximal vein partially occlusive thrombi is thought by some to indicate an increased risk of embolization, although there are no extensive studies confirming this concern.
Duplex sonography is not very reliable for distinguishing acute from subacute thrombus. However, chronic wall changes seen months or years after an episode of DVT are unlikely to be mistaken for an acute DVT. Repeat ultrasound examination following therapy may be useful in establishing a baseline given the possibility of DVT recurrence. It has been estimated that this rate can reach 5% per patient-year in the first 3 years following a first proximal DVT.